Enhancing Biochemical Methane Potential and Enrichment of Specific Electroactive Communities from Nixtamalization Wastewater using Granular Activated Carbon as a Conductive Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nejayote and Inoculum
2.2. BMP Test
2.3. GAC Conductivity
2.4. Analytical Methods
2.5. Microbial Community Analysis
2.6. Scanning Electron Microscopy (SEM)
3. Results
3.1. Nejayote and Inoculum
3.2. BMP Test
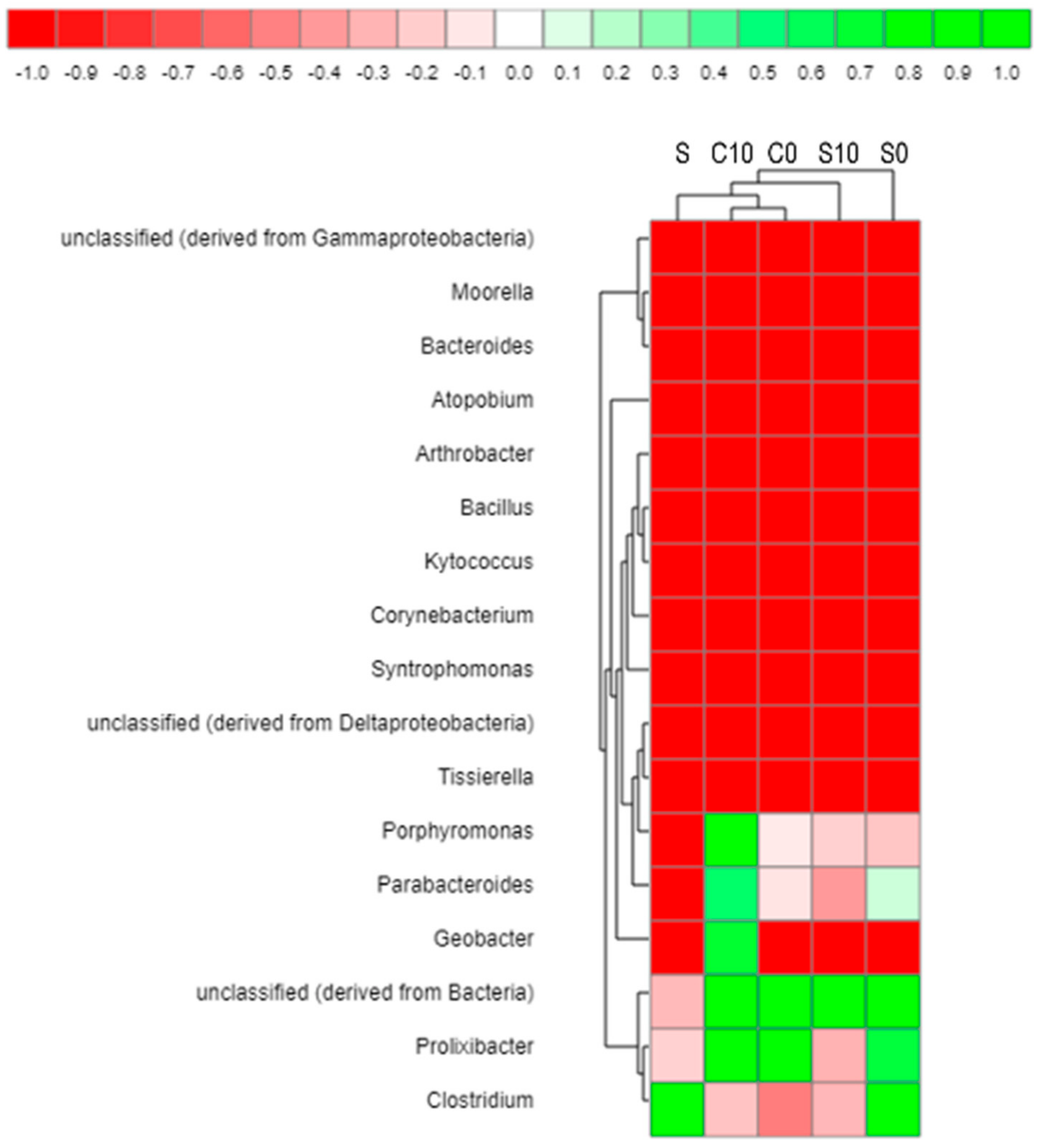
3.3. Microbial Community Analysis
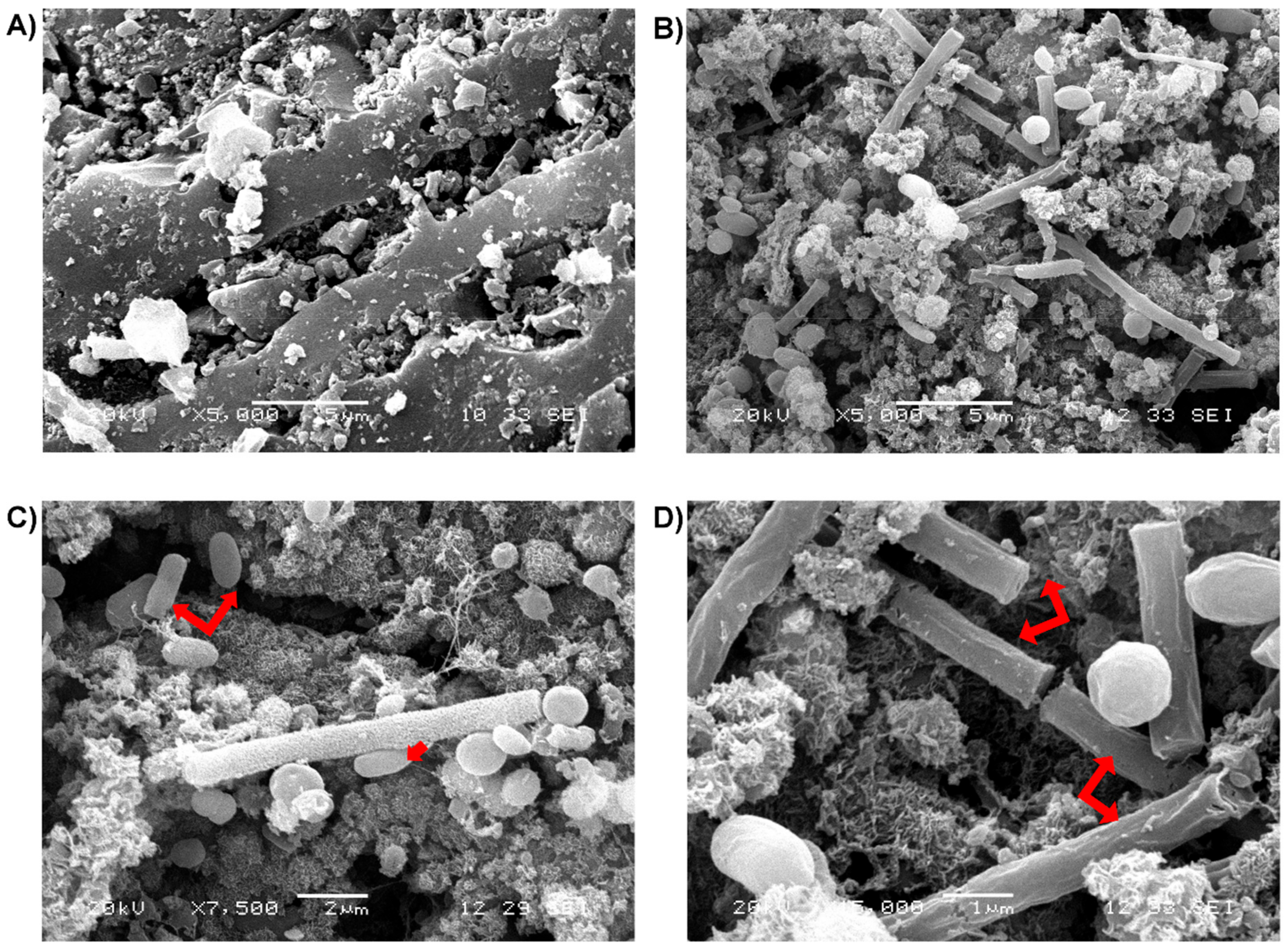
3.4. Scanning Electron Microscopy (SEM)
4. Discussion
4.1. Nejayote and Inoculum
4.2. BMP Test
4.3. GAC Conductivity
4.4. Redox Potential
4.5. Relationship Between Redox Potential, Methane Production and Archaea Communities
4.6. Microbial Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- España-Gamboa, E.; Domínguez-Maldonado, J.A.; Tapia-Tussell, R.; Chale-Canul, J.S.; Alzate-Gaviria, L. Corn industrial wastewater (nejayote): A promising substrate in Mexico for methane production in a coupled system (APCR-UASB). Environ. Sci. Pollut. Res. 2017, 25, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Salmerón-Alcocer, A.; Rodríguez-Mendoza, N.; Pineda-Santiago, V.; Cristiani-Urbina, E.; Juárez-Ramírez, C.; Ruiz-Ordaz, N.; Galíndez-Mayer, J. Aerobic treatment of maize-processing wastewater (nejayote) in a single-stream multi-stage bioreactor. J. Environ. Eng. Sci. 2003, 2, 401–406. [Google Scholar] [CrossRef]
- Rosentrater, K.A. A review of corn masa processing residues: Generation, properties, and potential utilization. Waste Manag. 2006, 26, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Valderrama-Bravo, C.; Gutiérrez-Cortez, E.; Contreras-Padilla, M.; Rojas-Molina, I.; Mosquera, J.C.; Rojas-Molina, A.; Beristain, F.; Rodríguez-García, M.E. Constant pressure filtration of lime water (nejayote) used to cook kernels in maize processing. J. Food Eng. 2012, 110, 478–486. [Google Scholar] [CrossRef]
- Meraz, K.A.S.; Vargas, S.M.P.; Maldonado, J.T.L.; Bravo, J.M.C.; Guzman, M.T.O.; Maldonado, E.A.L. Eco-friendly innovation for nejayote coagulation–flocculation process using chitosan: Evaluation through zeta potential measurements. Chem. Eng. J. 2016, 284, 536–542. [Google Scholar] [CrossRef]
- García-Zamora, J.L.; Sánchez-González, M.; Lozano, J.A.; Jáuregui, J.; Zayas, T.; Santacruz, V.; Hernández, F.; Torres, E. Enzymatic treatment of wastewater from the corn tortilla industry using chitosan as an adsorbent reduces the chemical oxygen demand and ferulic acid content. Process Biochem. 2015, 50, 125–133. [Google Scholar] [CrossRef]
- Argun, M.S.; Argun, M.E. Treatment and alternative usage possibilities of a special wastewater: Nejayote. J. Food Process Eng. 2018, 41, e12609. [Google Scholar] [CrossRef]
- Desloover, J.; De Clippeleir, H.; Boeckx, P.; Du Laing, G.; Colsen, J.; Verstraete, W.; Vlaeminck, S.E. Floc-based sequential partial nitritation and anammox at full scale with contrasting N2O emissions. Water Res. 2011, 45, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Massara, T.M.; Komesli, O.T.; Sozudogru, O.; Komesli, S.; Katsou, E. A Mini Review of the Techno-environmental Sustainability of Biological Processes for the Treatment of High Organic Content Industrial Wastewater Streams. Waste Biomass Valoriz. 2017, 8, 1665–1678. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Lyberatos, G. Food industry waste’s exploitation via anaerobic digestion and fermentative hydrogen production in an up-flow column reactor. Waste Biomass Valoriz. 2016, 7, 711–723. [Google Scholar] [CrossRef]
- Veluchamy, C.; Kalamdhad, A.S. Biochemical methane potential test for pulp and paper mill sludge with different food/microorganisms ratios and its kinetics. Int. Biodeterior. Biodegradation 2017, 117, 197–204. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; De la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.L.; Schmidt, J.E.; Angelidaki, I.; Marca, E.; la Cour Jansen, J.; Mosbæk, H.; Christensen, T.H. Method for determination of methane potentials of solid organic waste. Waste Manag. 2004, 24, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Quan, X.; Zhao, H. Evaluation on direct interspecies electron transfer in anaerobic sludge digestion of microbial electrolysis cell. Bioresour. Technol. 2016, 200, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Lee, S.-H.; Park, H.-D. Enrichment of specific electro-active microorganisms and enhancement of methane production by adding granular activated carbon in anaerobic reactors. Bioresour. Technol. 2016, 205, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and Potential of Direct Interspecies Electron Transfer in Anaerobic Digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Dubé, C.-D.; Guiot, S.R. Direct Interspecies Electron Transfer in Anaerobic Digestion: A Review. In Biogas Science and Technology; Guebitz, M.G., Bauer, A., Bochmann, G., Gronauer, A., Weiss, S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 101–115. ISBN 978-3-319-21993-6. [Google Scholar]
- Liu, F.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982–8989. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting Interspecies Electron Transfer with Biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Zhang, Y.; Holmes, D.E.; Dang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour. Technol. 2016, 209, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovley, D.R. Happy together: Microbial communities that hook up to swap electrons. ISME J. 2016, 11, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rotaru, A.-E.; Liu, F.; Philips, J.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour. Technol. 2014, 173, 82–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, Y.; Sun, D.; Woodard, T.L.; Wang, L.-Y.; Nevin, K.P.; Holmes, D.E. Stimulation of the anaerobic digestion of the dry organic fraction of municipal solid waste (OFMSW) with carbon-based conductive materials. Bioresour. Technol. 2017, 238, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.-Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Hesas, R.H.; Arami-Niya, A.; Daud, W.M.A.W.; Sahu, J.N. Preparation and characterization of activated carbon from apple waste by microwave-assisted phosphoric acid activation: Application in methylene blue adsorption. BioResources 2013, 8, 2950–2966. [Google Scholar]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S.; Abnisa, F.; Shafeeyan, M.S. Production of microporous palm shell based activated carbon for methane adsorption: Modeling and optimization using response surface methodology. Chem. Eng. Res. Des. 2012, 90, 776–784. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Yu, Q.; Dang, Y.; Li, Y.; Quan, X. Communities stimulated with ethanol to perform direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate. Water Res. 2016, 102, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.; Tinsley, C.J. Electrical conduction in magnetite. Phys. Status Solidi 1976, 33, 189–194. [Google Scholar] [CrossRef]
- Guo, K.; Soeriyadi, A.H.; Feng, H.; Prévoteau, A.; Patil, S.A.; Gooding, J.J.; Rabaey, K. Heat-treated stainless steel felt as scalable anode material for bioelectrochemical systems. Bioresour. Technol. 2015, 195, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Mo, J.; Steen, S.M.; Zhang, F.-Y.; Toops, T.J.; Brady, M.P.; Green, J.B. Electrochemical investigation of stainless steel corrosion in a proton exchange membrane electrolyzer cell. Int. J. Hydrogen Energy 2015, 40, 12506–12511. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Sun, D.; Ma, Y.; Qiu, B.; Guo, Z. Conductive polyaniline nanorods enhanced methane production from anaerobic wastewater treatment. Polymer 2017, 120, 236–243. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, S.; Li, X.; Zhang, M.; Zhou, J. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion. Bioresour. Technol. 2017, 224, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Wan, J.; Angelidaki, I.; Zhang, S.; Luo, G. iTRAQ quantitative proteomic analysis reveals the pathways for methanation of propionate facilitated by magnetite. Water Res. 2017, 108, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, C.; Yan, K.; Wu, J.; Zuo, J.; Wang, K. Anaerobic granule-based biofilms formation reduces propionate accumulation under high H2 partial pressure using conductive carbon felt particles. Bioresour. Technol. 2016, 216, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Sun, D.; Dang, Y.; Chen, H.; Zhao, Z.; Zhang, Y.; Holmes, D.E. Stimulation of methanogenesis in anaerobic digesters treating leachate from a municipal solid waste incineration plant with carbon cloth. Bioresour. Technol. 2016, 222, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Y. Conductive Fe3O4 nanoparticles accelerate syntrophic methane production from butyrate oxidation in two different lake sediments. Front. Microbiol. 2016, 7, 1316. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Shen, N.; Xiao, Y.; Chen, Y.; Sun, F.; Tyagi, V.K.; Zhou, Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresour. Technol. 2017, 239, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Yamada, C.; Kato, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Conductive iron oxides accelerate thermophilic methanogenesis from acetate and propionate. J. Biosci. Bioeng. 2015, 119, 678–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, L.; Tang, J.; Wang, Y.; Hu, M.; Zhou, S. Conductive iron oxide minerals accelerate syntrophic cooperation in methanogenic benzoate degradation. J. Hazard. Mater. 2015, 293, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 244, 698–707. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Poggi-Varaldo, H.M.; Valdés, L.; Esparza-Garcia, F.; Fernández-Villagómez, G. Solid substrate anaerobic co-digestion of paper mill sludge, biosolids, and municipal solid waste. Water Sci. Technol. 1997, 35, 197–204. [Google Scholar] [CrossRef]
- Wang, B.; Björn, A.; Strömberg, S.; Nges, I.A.; Nistor, M.; Liu, J. Evaluating the influences of mixing strategies on the Biochemical Methane Potential test. J. Environ. Manag. 2017, 185, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Montes, J.A.; Rico, J.L.; Rico, C. Influence of headspace pressure on methane production in Biochemical Methane Potential (BMP) tests. Waste Manag. 2016, 48, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowler, N. Four-point potential drop measurements for materials characterization. Meas. Sci. Technol. 2011, 22, 12001. [Google Scholar] [CrossRef]
- Wirth, R.; Kovács, E.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnol. Biofuels 2012, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Trejo-González, A.; Fería-Morales, A.; Wild-Altamirano, C. The role of lime in the alkaline treatment of corn for tortilla preparation. Adv. Chem. 1982, 198, 245–263. [Google Scholar]
- González-Martinez, S. Biological treatability of the wastewaters from the alkaline cooking of maize (Indian corn). Environ. Technol. 1984, 5, 365–372. [Google Scholar] [CrossRef]
- Eddy, M. Wastewater Engineering: Treatment, Disposal and Reuse; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Cruz Viggi, C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014, 48, 7536–7543. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhao, Z.; Ngo, H.H.; Guo, W.; Zhou, J.; Peng, L.; Ni, B.-J. A modeling approach to direct interspecies electron transfer process in anaerobic transformation of ethanol to methane. Environ. Sci. Pollut. Res. 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Phillips, E.J.P. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [PubMed]
- Kamagata, Y.; Kawasaki, H.; Oyaizu, H.; Nakamura, K.; Mikami, E.; Endo, G.; Koga, Y.; Yamasato, K. Characterization of three thermophilic strains of Methanothrix (“Methanosaeta”) thermophila sp. nov. and rejection of Methanothrix (“Methanosaeta”) thermoacetophila. Int. J. Syst. Evol. Microbiol. 1992, 42, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Mendívil, M.H.; Gallardo-Navarro, Y.T.; Torres, P.I.; Ramírez Wong, B. Effect of processing conditions on instrumental evaluation of nixtamal hardness of corn. J. Texture Stud. 2008, 39, 252–266. [Google Scholar] [CrossRef]
- Rosentrater, K.A.; Flores, R.A.; Richard, T.L.; Bern, C.J. Physical and nutritional properties of corn masa by-product streams. Appl. Eng. Agric. 1999, 15, 515–523. [Google Scholar] [CrossRef]
- Krishnan, R.; Ríos, R.; Salinas, N.; Durán-de-Bazúa, C. Treatment of Maize Processing Industry Wastewater by Percolating Columns. Environ. Technol. 1998, 19, 417–424. [Google Scholar] [CrossRef]
- Chen, Y.; Kurt, S.; Creamer, J.J.C. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2007, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Kumar, S.; Ojha, C.S.P. Nutrient requirement for UASB process: A review. Biochem. Eng. J. 1999, 3, 35–54. [Google Scholar] [CrossRef]
- Omil, F.; Lens, P.; Visser, A.; Hulshoff Pol, L.W.; Lettinga, G. Long-term competition between sulfate reducing and methanogenic bacteria in UASB reactors treating volatile fatty acids. Biotechnol. Bioeng. 1998, 57, 676–685. [Google Scholar] [CrossRef]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 0471468959. [Google Scholar]
- Lettinga, G.; Hobma, S.W.; Pol, L.W.H.; De Zeeuw, W.; De Jong, P.; Grin, P.; Roersma, R. Design operation and economy of anaerobic treatment. Water Sci. Technol. 1983, 15, 177–195. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Yang, Y.; Quan, X.; Zhao, Z. Potentially direct interspecies electron transfer of methanogenesis for syntrophic metabolism under sulfate reducing conditions with stainless steel. Bioresour. Technol. 2017, 234, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Matsumoto, N.; Morita, M.; Sasaki, K.; Ohmura, N. Electrochemical control of redox potential affects methanogenesis of the hydrogenotrophic methanogen Methanothermobacter thermautotrophicus. Lett. Appl. Microbiol. 2013, 56, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.F.; Martins, G.; Melle-Franco, M.; Serpa, R.; Stams, A.J.M.; Cavaleiro, A.J.; Pereira, M.A.; Alves, M.M. Carbon nanotubes accelerate methane production in pure cultures of methanogens and in a syntrophic coculture. Environ. Microbiol. 2017, 19, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Thrash, J.C.; Coates, J.D. Direct and indirect electrical stimulation of microbial metabolism. Environ. Sci. Technol. 2008, 42, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Zinder, S.H. Physiological ecology of methanogens. In Methanogenesis; Springer: Boston, MA, USA 1993; pp. 128–206. [Google Scholar]
- Xu, S.; He, C.; Luo, L.; Lü, F.; He, P.; Cui, L. Comparing activated carbon of different particle sizes on enhancing methane generation in upflow anaerobic digester. Bioresour. Technol. 2015, 196, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Liu, L.; Sun, D.; Ren, N.; Lee, D.-J. Isolation of Fe (III)-reducing fermentative bacterium Bacteroides sp. W7 in the anode suspension of a microbial electrolysis cell (MEC). Int. J. Hydrogen Energy 2010, 35, 3178–3182. [Google Scholar] [CrossRef]
- Wu, D.; Wang, T.; Huang, X.; Dolfing, J.; Xie, B. Perspective of harnessing energy from landfill leachate via microbial fuel cells: Novel biofuels and electrogenic physiologies. Appl. Microbiol. Biotechnol. 2015, 99, 7827–7836. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Nevin, K.P.; Woodard, T.L.; Peacock, A.D.; Lovley, D.R. Prolixibacter bellariivorans gen. nov., sp. nov., a sugar-fermenting, psychrotolerant anaerobe of the phylum Bacteroidetes, isolated from a marine-sediment fuel cell. Int. J. Syst. Evol. Microbiol. 2007, 57, 701–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Jiang, L.; Wang, Q.; Quan, X.; Yang, J.; Chen, L. Cobalt recovery with simultaneous methane and acetate production in biocathode microbial electrolysis cells. Chem. Eng. J. 2014, 253, 281–290. [Google Scholar] [CrossRef]
- Hodgson, D.M.; Smith, A.; Dahale, S.; Stratford, J.P.; Li, J.V.; Grüning, A.; Bushell, M.E.; Marchesi, J.R.; Avignone Rossa, C. Segregation of the anodic microbial communities in a microbial fuel cell cascade. Front. Microbiol. 2016, 7, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toczyłowska-Mamińska, R.; Szymona, K.; Król, P.; Gliniewicz, K.; Pielech-Przybylska, K.; Kloch, M.; Logan, B.E. Evolving Microbial Communities in Cellulose-Fed Microbial Fuel Cell. Energies 2018, 11, 124. [Google Scholar] [CrossRef]



| Parameter | N | N0 | N10 |
|---|---|---|---|
| pH | 7.3 ± 0.11 | 7.2 ± 0.03 | 7.3 ± 0.02 |
| Redox potential (mV) | −223 ± 5 | −467 ± 1 | −471 ± 2 |
| Acetic acid (mg·L−1) | 203 ± 12 | 139 ± 9 | 109 ± 8 |
| Butyric acid (mg·L−1) | 91 ± 5 | ||
| COD (mg·L−1) | 532 ± 10 | 307 ± 21 | 218 ± 14 |
| Parameter | Results | Other Studies |
|---|---|---|
| pH | 10.2 | 6.3–11.6 [1,3,4,5,62,63] |
| COD (mg·L−1) | 15,433 ± 826 | 3430–40,058 [1,4,5,63] |
| NH3-N (mg·L−1) | 4.65 ± 0.05 | 2 [1] |
| TN (mg·L−1) | 95.33 ± 3.7 | 209–428 [1,5] |
| SO4−2 (mg·L−1) | 22.5 ± 2.5 | 13 [1] |
| PO4−3 (mg·L−1) | 58.75 ± 1.2 | 7.6–1321 [1,5] |
| TA (mgCaCO3·L−1) | 1799 ± 116 | 5768 [4] |
| TSS (mg·L−1) | 2676 ± 512 | 1810–8340 [3,4] |
| TS% | 1.22 ± 0.01 | 0.34–2.5 [1,4,63] |
| VS% | 0.84 ± 0.01 | 0.24–1.55 [1,63] |
| VS/TS | 0.69 ± 0.01 | 0.70 [63] |
| Reactor Volume (mL) | Substrate | Conductive Material | Methane Production Increase (%) | Time (Days) | Reference |
|---|---|---|---|---|---|
| 120 | Nejayote | GAC | 54 | 30 | This study |
| 120 | Nejayote | GAC | 34 | 30 | This study |
| 250 | Ethanol | Graphene | 25 | 12 | [38] |
| 500 | Synthetic wastewater | GAC | 86 | 43 | [17] |
| 120 | Synthetic wastewater | Magnetite | 32 | 20 | [56] |
| 250 | Glucose | Graphene | 51 | 15 | [36] |
| 250 | Sludge treatment plant | GAC | 17 | 20 | [33] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero, D.; Rico, C.; Canto-Canché, B.; Domínguez-Maldonado, J.A.; Tapia-Tussell, R.; Cortes-Velazquez, A.; Alzate-Gaviria, L. Enhancing Biochemical Methane Potential and Enrichment of Specific Electroactive Communities from Nixtamalization Wastewater using Granular Activated Carbon as a Conductive Material. Energies 2018, 11, 2101. https://doi.org/10.3390/en11082101
Valero D, Rico C, Canto-Canché B, Domínguez-Maldonado JA, Tapia-Tussell R, Cortes-Velazquez A, Alzate-Gaviria L. Enhancing Biochemical Methane Potential and Enrichment of Specific Electroactive Communities from Nixtamalization Wastewater using Granular Activated Carbon as a Conductive Material. Energies. 2018; 11(8):2101. https://doi.org/10.3390/en11082101
Chicago/Turabian StyleValero, David, Carlos Rico, Blondy Canto-Canché, Jorge Arturo Domínguez-Maldonado, Raul Tapia-Tussell, Alberto Cortes-Velazquez, and Liliana Alzate-Gaviria. 2018. "Enhancing Biochemical Methane Potential and Enrichment of Specific Electroactive Communities from Nixtamalization Wastewater using Granular Activated Carbon as a Conductive Material" Energies 11, no. 8: 2101. https://doi.org/10.3390/en11082101





