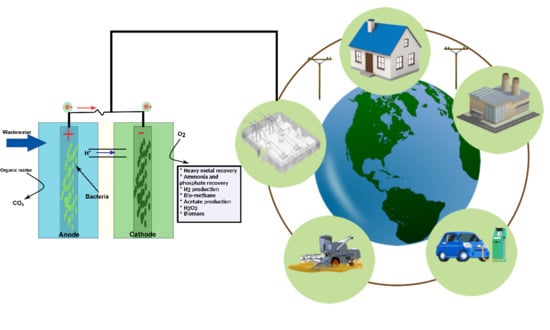
Electro-Microbiology as a Promising Approach Towards Renewable Energy and Environmental Sustainability
Abstract
:1. Introduction
2. Microbe-Electrode Interaction
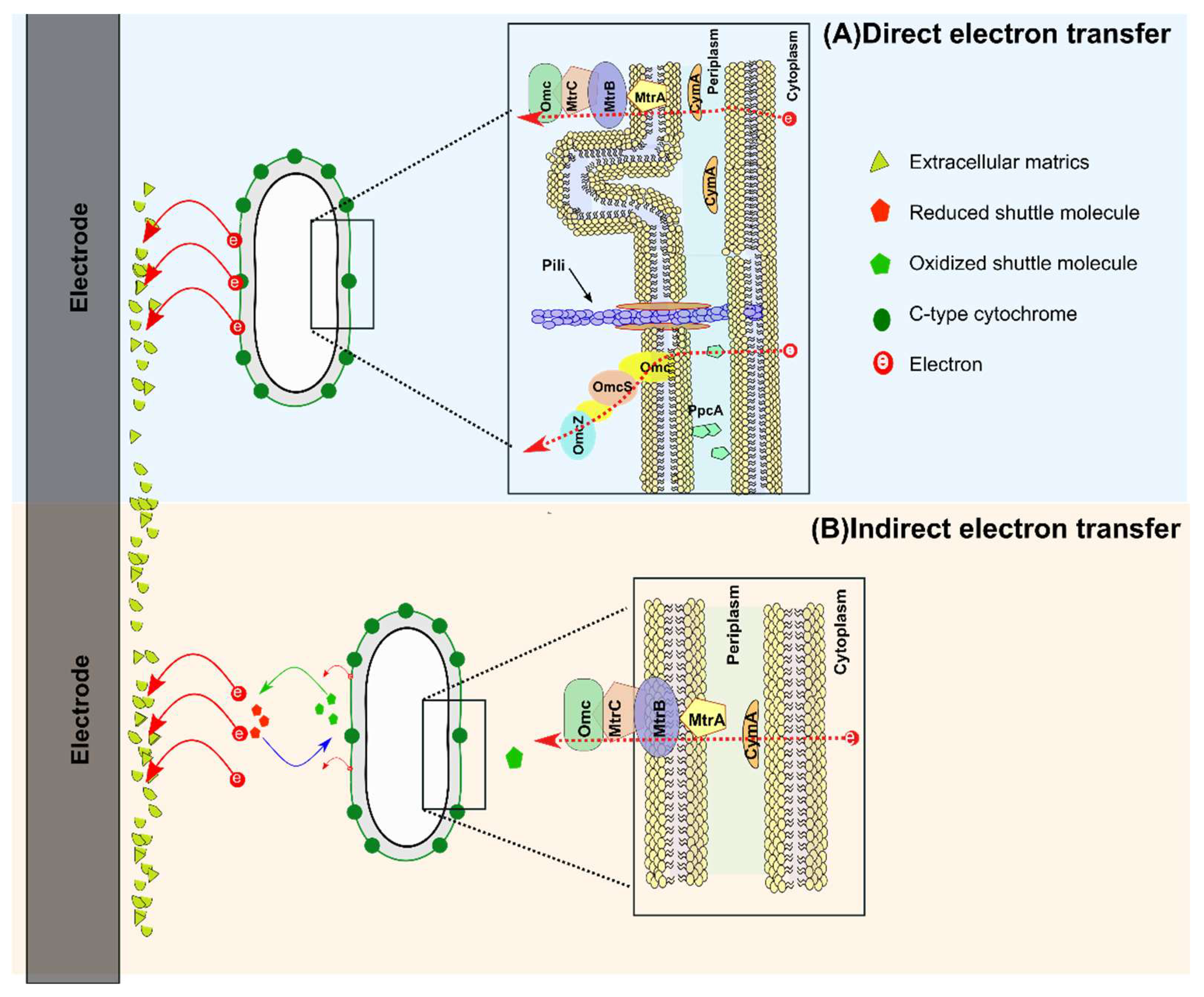
2.1. Mechanism of Electron Transfer
2.1.1. Direct Electron Transfer (DET)
2.1.2. Indirect Electron Transfer (IET)
3. Role of Characterization Techniques in the Advancement of Electro-Microbiology
3.1. Fundamental and Culturing Techniques
3.2. Molecular Techniques for Characterization of Electroactive Bacteria
3.3. Electrochemical Characterization of Microbial Communities
3.3.1. Cyclic Voltammetry (CV)
3.3.2. Electrochemical Impedance Spectroscopy (EIS)
3.3.3. Square Wave Voltammetry (SWV)
3.3.4. Chronoamperometry (CA)
3.3.5. Differential Pulse Voltammetry (DPV)
4. Electro-Microbiology and Environmental Sustainability
4.1. Simultaneous Electricity Generation & Wastewater Treatment by MFCs
4.2. Resource Recovery and Sustainability
4.2.1. Metal Recovery
4.2.2. Ammonia and Phosphorus Recovery
4.2.3. Biosynthesis Prospects of Electro-Microbiology
Biomethane
Biohydrogen
Acetate
Hydrogen Peroxide
Biomass
5. Future Perspectives
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Chabert, N.; Ali, O.A.; Achouak, W. All ecosystems potentially host electrogenic bacteria. Bioelectrochem. 2015, 106, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Nealson, K.H. Bioelectricity (electromicrobiology) and sustainability. Microb. Biotechnol. 2017, 10, 1114–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. Ser. B Contain. Papers Biol. Character 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [PubMed]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (mfcs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Rittmann, B.E. Opportunities for renewable bioenergy using microorganisms. Biotechnol. Bioeng. 2008, 100, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Sekar, N.; Ramasamy, R.P. Electrochemical impedance spectroscopy for microbial fuel cell characterization. J. Microb. Biochem. Technol. 2013. [Google Scholar] [CrossRef]
- Zhi, W.; Ge, Z.; He, Z.; Zhang, H. Methods for understanding microbial community structures and functions in microbial fuel cells: A review. Bioresour. Technol. 2014, 171, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Dolch, K.; Danzer, J.; Kabbeck, T.; Bierer, B.; Erben, J.; Förster, A.H.; Maisch, J.; Nick, P.; Kerzenmacher, S.; Gescher, J. Characterization of microbial current production as a function of microbe-electrode-interaction. Bioresour. Technol. 2014, 157, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Nevin, K.P. A shift in the current: New applications and concepts for microbe-electrode electron exchange. Curr. Opin. Biotechnol. 2011, 22, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Harnisch, F.; Freguia, S. A basic tutorial on cyclic voltammetry for the investigation of electroactive microbial biofilms. Chemistry–An Asian Journal 2012, 7, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Leang, C.; Franks, A.E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Specific localization of the c-type cytochrome omcz at the anode surface in current-producing biofilms of geobacter sulfurreducens. Environ. Microbiol. Rep. 2011, 3, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hsu, L.H.-H.; Kavanagh, P.; Barrière, F.; Lens, P.N.; Lapinsonnière, L.; Schröder, U.; Jiang, X.; Leech, D. The ins and outs of microorganism-electrode electron transfer reactions. Nat. Rev. Chem. 2017, 1. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Huang, L.; You, L.; Zhuang, L.; Zhou, S. Electrochemical and spectroscopic insights into the mechanisms of bidirectional microbe-electrode electron transfer in geobacter soli biofilms. Electrochem. Commun. 2017, 77, 93–97. [Google Scholar] [CrossRef]
- Okamoto, A.; Nakamura, R.; Hashimoto, K. In-vivo identification of direct electron transfer from shewanella oneidensis mr-1 to electrodes via outer-membrane omca–mtrcab protein complexes. Electrochim. Acta. 2011, 56, 5526–5531. [Google Scholar] [CrossRef]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Qian, X.; Morgado, L.; Kim, B.-C.; Mester, T.; Izallalen, M.; Salgueiro, C.A.; Lovley, D.R. Purification and characterization of omcz, an outer-surface, octaheme c-type cytochrome essential for optimal current production by geobacter sulfurreducens. Appl. Environ. Microbiol. 2010, 76, 3999–4007. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 2018, 11. [Google Scholar] [CrossRef]
- Lovley, D.R. Syntrophy goes electric: Direct interspecies electron transfer. Annu. Rev. Microbiol. 2017, 71, 643–664. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Shrestha, P.M.; Walker, D.J.; Dang, Y.; Nevin, K.P.; Woodard, T.L.; Lovley, D.R. Metatranscriptomic evidence for direct interspecies electron transfer between geobacter and methanothrix species in methanogenic rice paddy soils. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, Y.; Guo, C.X.; Lim, S.; Song, H.; Li, C.M. Graphene/carbon cloth anode for high-performance mediatorless microbial fuel cells. Bioresour. Technol. 2012, 114, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Squier, T.C.; Zachara, J.M.; Fredrickson, J.K. Respiration of metal (hydr) oxides by shewanella and geobacter: A key role for multihaem c-type cytochromes. Mol. Microbiol. 2007, 65, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Evidence for involvement of an electron shuttle in electricity generation by geothrix fermentans. Appl. Environ. Microbiol. 2005, 71, 2186–2189. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.-Y.; Shi, D.-Y.; Chen, Y.-L.; Jiao, F.; Lin, X.; Zhou, J.; Wang, S.-Y.; Yong, Y.-C.; Sun, Y.-M.; OuYang, P.-K. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour. Technol. 2014, 152, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, Y.; Hu, Y.; Cao, B.; Rice, S.A.; Kjelleberg, S.; Song, H. Enhancing bidirectional electron transfer of shewanella oneidensis by a synthetic flavin pathway. ACS Synth. Biol. 2015, 4, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, G.; Bakonyi, P.; Zhen, G.; Sivagurunathan, P.; Koók, L.; Kim, S.-H.; Tóth, G.; Nemestóthy, N.; Bélafi-Bakó, K. Microbial electrochemical systems for sustainable biohydrogen production: Surveying the experiences from a start-up viewpoint. Renew. Sustain. Energy Rev. 2017, 70, 589–597. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Qu, Y.; Feng, Y. Enhanced shewanella oneidensis mr-1 anode performance by adding fumarate in microbial fuel cell. Chem. Eng. J. 2017, 328, 697–702. [Google Scholar] [CrossRef]
- Okamoto, A.; Saito, K.; Inoue, K.; Nealson, K.H.; Hashimoto, K.; Nakamura, R. Uptake of self-secreted flavins as bound cofactors for extracellular electron transfer in geobacter species. Energy Environ. Sci. 2014, 7, 1357–1361. [Google Scholar] [CrossRef]
- Lovley, D.R. Long-range electron transport to fe (iii) oxide via pili with metallic-like conductivity. Biochem. Soc. Trans. 2012, 40, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Kracke, F.; Vassilev, I.; Krömer, J.O. Microbial electron transport and energy conservation–the foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of organic compounds from carbon dioxide catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Bosire, E.M.; Blank, L.M.; Rosenbaum, M.A. Strain and substrate dependent redox mediator and electricity production by pseudomonas aeruginosa. Appl. Environ. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.L.; Aklujkar, M.; Leang, C.; Nevin, K.P.; Lovley, D. A genetic system for geobacter metallireducens: Role of the flagellin and pilin in the reduction of fe (iii) oxide. Environ. Microbiol. Rep. 2012, 4, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; Myers, C.R. Role for outer membrane cytochromes omca and omcb of shewanella putrefaciens mr-1 in reduction of manganese dioxide. Appl. Environ. Microbiol. 2001, 67, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Venkidusamy, K.; Megharaj, M. A novel electrophototrophic bacterium rhodopseudomonas palustris strain rp2, exhibits hydrocarbonoclastic potential in anaerobic environments. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, X.; Zhang, W.; Hu, M.; Li, F. Fe (iii) oxides accelerate microbial nitrate reduction and electricity generation by klebsiella pneumoniae l17. J. Colloid. Interface Sci. 2014, 423, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl. Environ. Microbiol. 2012, 78, 8412–8420. [Google Scholar] [CrossRef] [PubMed]
- Freguia, S.; Masuda, M.; Tsujimura, S.; Kano, K. Lactococcus lactis catalyses electricity generation at microbial fuel cell anodes via excretion of a soluble quinone. Bioelectrochemistry 2009, 76, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Coursolle, D.; Gralnick, J.A. Modularity of the mtr respiratory pathway of shewanella oneidensis strain mr-1. Mol. Microbiol. 2010, 77, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Le Laz, S.; Kpebe, A.; Lorquin, J.; Brugna, M.; Rousset, M. H 2-dependent azoreduction by shewanella oneidensis mr-1: Involvement of secreted flavins and both [ni–fe] and [fe–fe] hydrogenases. Appl. Microbiol. Biotechnol. 2014, 98, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Ishikawa, M.; Hashimoto, K.; Nakanishi, S. Molecular design of cytocompatible amphiphilic redox-active polymers for efficient extracellular electron transfer. Bioelectrochemistry 2017, 114, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Slade, R.C.; Varcoe, J.R. Techniques for the study and development of microbial fuel cells: An electrochemical perspective. Chem. Soc. Rev. 2009, 38, 1926–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; Li, J.; Qin, D.; Chen, L.; Zhao, F.; Chen, S.; Hu, H.; Yu, C.-P. Characterization of exoelectrogenic bacteria enterobacter strains isolated from a microbial fuel cell exposed to copper shock load. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, E.S.; Curtis, T.P.; Woodcock, S.; Dolfing, J. Quantification of effective exoelectrogens by most probable number (mpn) in a microbial fuel cell. Bioresour. Technol. 2016, 218, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Wrighton, K.C.; Agbo, P.; Warnecke, F.; Weber, K.A.; Brodie, E.L.; DeSantis, T.Z.; Hugenholtz, P.; Andersen, G.L.; Coates, J.D. A novel ecological role of the firmicutes identified in thermophilic microbial fuel cells. ISME J. 2008, 2, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Sacco, N.J.; Bonetto, M.C.; Cortón, E. Isolation and characterization of a novel electrogenic bacterium, dietzia sp. Rnv-4. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Deng, D.; Zhang, D.; Chen, Q.; Kang, J.; Fan, N.; Liu, Y. Microbial electricity generation and isolation of exoelectrogenic bacteria based on petroleum hydrocarbon-contaminated soil. Electroanalysis 2016, 28, 1510–1516. [Google Scholar] [CrossRef]
- Zuo, Y.; Xing, D.; Regan, J.M.; Logan, B.E. Isolation of the exoelectrogenic bacterium ochrobactrum anthropi yz-1 by using a u-tube microbial fuel cell. Appl. Environ. Microbiol. 2008, 74, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, Y.; Quan, X.; Chen, S. Anaerobic biodecolorization of ao7 by a newly isolated fe (iii)-reducing bacterium sphingomonas strain dj. J. Chem. Technol. Biotechnol. 2015, 90, 158–165. [Google Scholar] [CrossRef]
- Xing, D.; Zuo, Y.; Cheng, S.; Regan, J.M.; Logan, B.E. Electricity generation by rhodopseudomonas palustris dx-1. Environ. Sci. Technol. 2008, 42, 4146–4151. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Call, D.; Wang, A.; Cheng, S.; Logan, B.E. Geobacter sp. Sd-1 with enhanced electrochemical activity in high-salt concentration solutions. Environ. Microbiol. Rep. 2014, 6, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lee, D.-J.; Wang, A.; Ren, N.; Su, A.; Lai, J.-Y. Isolation of fe (iii)-reducing bacterium, citrobacter sp. Lar-1, for startup of microbial fuel cell. Int. J. Hydrogen Energy 2016, 41, 4498–4503. [Google Scholar] [CrossRef]
- Nercessian, O.; Parot, S.; Délia, M.-L.; Bergel, A.; Achouak, W. Harvesting electricity with geobacter bremensis isolated from compost. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Wang, Y.; Qin, D.; Yang, G.; Zhou, S. Bacillus sediminis sp. Nov., isolated from an electroactive biofilm. Antonie Van Leeuwenhoek 2013, 104, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, B.H.; Kim, H.S.; Kim, H.J.; Kim, G.T.; Kim, M.; Chang, I.S.; Park, Y.K.; Chang, H.I. A novel electrochemically active and fe (iii)-reducing bacterium phylogenetically related to clostridium butyricum isolated from a microbial fuel cell. Anaerobe 2001, 7, 297–306. [Google Scholar] [CrossRef]
- Finneran, K.T.; Johnsen, C.V.; Lovley, D.R. Rhodoferax ferrireducens sp. Nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of fe (iii). Int. J. Syst. Evol. Microbiol. 2003, 53, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Hyun, M.; Chang, I.; Kim, H.; Park, H.; Kim, B.; Kim, S.; Wimpenny, J.; Weightman, A.J. Dissimilatory fe (iii) reduction by an electrochemically active lactic acid bacterium phylogenetically related to enterococcus gallinarum isolated from submerged soil. J. Appl. Microbiol. 2005, 99, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.A.; Jung, S.J.; Phung, N.T.; Lee, J.; Chang, I.S.; Kim, B.H.; Yi, H.; Chun, J. A novel electrochemically active and fe (iii)-reducing bacterium phylogenetically related to aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Lett. 2003, 223, 129–134. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, X.; Li, X.; Zhang, Y.; Jiang, L.; Li, J.; Guan, Z.; Cai, Y.; Liao, X. The rhizospheric microbial community structure and diversity of deciduous and evergreen forests in taihu lake area, china. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Baranitharan, E.; Khan, M.R.; Prasad, D.; Teo, W.F.A.; Tan, G.Y.A.; Jose, R. Effect of biofilm formation on the performance of microbial fuel cell for the treatment of palm oil mill effluent. Bioprocess Biosyst. Eng. 2015, 38, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Roy, S.; Das, D. Genomic and proteomic approaches for dark fermentative biohydrogen production. Renew. Sustain. Energy Rev. 2016, 56, 1308–1321. [Google Scholar] [CrossRef]
- Parameswaran, P.; Torres, C.I.; Lee, H.-S.; Rittmann, B.E.; Krajmalnik-Brown, R. Hydrogen consumption in microbial electrochemical systems (mxcs): The role of homo-acetogenic bacteria. Bioresour. Technol. 2011, 102, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kiely, P.D.; Cusick, R.; Call, D.F.; Selembo, P.A.; Regan, J.M.; Logan, B.E. Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters. Bioresour. Technol. 2011, 102, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.i.; Suzuki, S.; Yamanaka, Y.; Wu, A.; Nealson, K.H.; Bretschger, O. Population dynamics of electrogenic microbial communities in microbial fuel cells started with three different inoculum sources. Bioelectrochemistry 2017. [Google Scholar] [CrossRef] [PubMed]
- Paitier, A.; Godain, A.; Lyon, D.; Haddour, N.; Vogel, T.M.; Monier, J.-M. Microbial fuel cell anodic microbial population dynamics during mfc start-up. Biosens. Bioelectron. 2017, 92, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Hungate, B.A.; Mau, R.L.; Schwartz, E.; Caporaso, J.G.; Dijkstra, P.; van Gestel, N.; Koch, B.J.; Liu, C.M.; McHugh, T.A.; Marks, J.C. Quantitative microbial ecology through stable isotope probing. Appl. Environ. Microbiol. 2015, 81, 7570–7581. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.i.; Suzuki, S.; Norden-Krichmar, T.M.; Tenney, A.; Chain, P.S.; Scholz, M.B.; Nealson, K.H.; Bretschger, O. A novel metatranscriptomic approach to identify gene expression dynamics during extracellular electron transfer. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- McLean, J.S.; Wanger, G.; Gorby, Y.A.; Wainstein, M.; McQuaid, J.; Ishii, S.i.; Bretschger, O.; Beyenal, H.; Nealson, K.H. Quantification of electron transfer rates to a solid phase electron acceptor through the stages of biofilm formation from single cells to multicellular communities. Environ. Sci. Technol. 2010, 44, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Kim, B.-C.; Glaven, R.H.; Johnson, J.P.; Woodard, T.L.; Methé, B.A.; DiDonato Jr, R.J.; Covalla, S.F.; Franks, A.E.; Liu, A. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in geobacter sulfurreducens fuel cells. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Marsili, E.; Rollefson, J.B.; Baron, D.B.; Hozalski, R.M.; Bond, D.R. Microbial biofilm voltammetry: Direct electrochemical characterization of catalytic electrode-attached biofilms. Appl. Environ. Microbiol. 2008, 74, 7329–7337. [Google Scholar] [CrossRef] [PubMed]
- Strycharz-Glaven, S.M.; Snider, R.M.; Guiseppi-Elie, A.; Tender, L.M. On the electrical conductivity of microbial nanowires and biofilms. Energy Environ. Sci. 2011, 4, 4366–4379. [Google Scholar] [CrossRef]
- Manohar, A.K.; Bretschger, O.; Nealson, K.H.; Mansfeld, F. The use of electrochemical impedance spectroscopy (eis) in the evaluation of the electrochemical properties of a microbial fuel cell. Bioelectrochemistry 2008, 72, 149–154. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Mansfeld, F. Exploring the use of electrochemical impedance spectroscopy (eis) in microbial fuel cell studies. Energy Environ. Sci. 2009, 2, 215–219. [Google Scholar] [CrossRef]
- Borole, A.P.; Aaron, D.; Hamilton, C.Y.; Tsouris, C. Understanding long-term changes in microbial fuel cell performance using electrochemical impedance spectroscopy. Environ. Sci. Technol. 2010, 44, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Herrera, D.; Pacheco-Catalan, D.; Valdez-Ojeda, R.; Canto-Canche, B.; Dominguez-Benetton, X.; Domínguez-Maldonado, J.; Alzate-Gaviria, L. Characterization of anode and anolyte community growth and the impact of impedance in a microbial fuel cell. BMC Biotechnol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Babauta, J.T.; Beyenal, H. Use of a small overpotential approximation to analyze geobacter sulfurreducens biofilm impedance. J. Power Sources 2017, 356, 549–555. [Google Scholar] [CrossRef]
- Qiao, Y.-J.; Qiao, Y.; Zou, L.; Wu, X.-S.; Liu, J.-H. Biofilm promoted current generation of pseudomonas aeruginosa microbial fuel cell via improving the interfacial redox reaction of phenazines. Bioelectrochemistry 2017. [Google Scholar] [CrossRef] [PubMed]
- Cercado-Quezada, B.; Delia, M.-L.; Bergel, A. Testing various food-industry wastes for electricity production in microbial fuel cell. Bioresour. Technol. 2010, 101, 2748–2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricke, K.; Harnisch, F.; Schröder, U. On the use of cyclic voltammetry for the study of anodic electron transfer in microbial fuel cells. Energy Environ. Sci. 2008, 1, 144–147. [Google Scholar] [CrossRef]
- Chen, S.; Patil, S.A.; Schröder, U. A high-performance rotating graphite fiber brush air-cathode for microbial fuel cells. Appl. Energy 2018, 211, 1089–1094. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Chen, C.-C.; Lin, Y.-Q.; Hsiao, C.-F.; Tsai, C.-H.; Hsieh, M.-H. Status of waste tires’ recycling for material and energy resources in taiwan. J. Mater. Cycles. Waste Manag. 2017, 19, 1288–1294. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Zhao, F.; Quaas, M.; Wulff, H.; Schröder, U.; Scholz, F. Evaluation of catalytic properties of tungsten carbide for the anode of microbial fuel cells. Appl. Catal. B Environ. 2007, 74, 261–269. [Google Scholar] [CrossRef]
- Zou, L.; Qiao, Y.; Zhong, C.; Li, C.M. Enabling fast electron transfer through both bacterial outer-membrane redox centers and endogenous electron mediators by polyaniline hybridized large-mesoporous carbon anode for high-performance microbial fuel cells. Electrochim. Acta. 2017, 229, 31–38. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A star* d report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Jafar Ali, S.Z.; Ali, N. Green synthesis of metal nanoparticles by microorganisms; a current prospective. J. Nanoanal 2015, 2, 32–38. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kiely, P.D.; Logan, B.E. A monetary comparison of energy recovered from microbial fuel cells and microbial electrolysis cells fed winery or domestic wastewaters. Int. J. Hydrogen Energy 2010, 35, 8855–8861. [Google Scholar] [CrossRef]
- Liang, B.; Kong, D.; Ma, J.; Wen, C.; Yuan, T.; Lee, D.-J.; Zhou, J.; Wang, A. Low temperature acclimation with electrical stimulation enhance the biocathode functioning stability for antibiotics detoxification. Water Res. 2016, 100, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Liu, Y.; Ma, J.; Zhao, F. Rapid degradation of sulphamethoxazole and the further transformation of 3-amino-5-methylisoxazole in a microbial fuel cell. Water Res. 2016, 88, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Kong, F.; Zheng, H.; Yin, J.; Cao, D.; Ren, Y.; Wang, G. Simultaneous processes of electricity generation and ceftriaxone sodium degradation in an air-cathode single chamber microbial fuel cell. J. Power Sources 2011, 196, 2567–2572. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, S.; Sun, D.; Huang, H.; Chen, J.; Cen, K. Inhibition of microbial growth on air cathodes of single chamber microbial fuel cells by incorporating enrofloxacin into the catalyst layer. Biosens. Bioelectron. 2015, 72, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, C.; Li, Y.; Gao, J.; Yu, C.-P. Enhancement of emerging contaminants removal using fenton reaction driven by h2o2-producing microbial fuel cells. Chem. Eng. J. 2017, 307, 679–686. [Google Scholar] [CrossRef]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Oh, S.; Logan, B.E. Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res. 2005, 39, 4673–4682. [Google Scholar] [CrossRef] [PubMed]
- Mohanakrishna, G.; Mohan, S.V.; Sarma, P. Bio-electrochemical treatment of distillery wastewater in microbial fuel cell facilitating decolorization and desalination along with power generation. J. Hazard Mater. 2010, 177, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ni, J. Treatment of wastewater from dioscorea zingiberensis tubers used for producing steroid hormones in a microbial fuel cell. Bioresour. Technol. 2011, 102, 2731–2735. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Parnas, R.; Li, B. Bioenergy production from glycerol in hydrogen producing bioreactors (hpbs) and microbial fuel cells (mfcs). Int. J. Hydrogen Energy 2011, 36, 3853–3861. [Google Scholar] [CrossRef]
- Sun, J.; Hu, Y.-Y.; Bi, Z.; Cao, Y.-Q. Simultaneous decolorization of azo dye and bioelectricity generation using a microfiltration membrane air-cathode single-chamber microbial fuel cell. Bioresour. Technol. 2009, 100, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, A.; Li, J.; He, B. A continuous process for biodiesel production in a fixed bed reactor packed with cation-exchange resin as heterogeneous catalyst. Bioresour. Technol. 2011, 102, 3607–3609. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zhou, S.-G.; Zhuang, L.; Zhang, J.-T.; Ni, J.-R. Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem. Eng. J. 2009, 43, 246–251. [Google Scholar] [CrossRef]
- Huang, L.; Logan, B.E. Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl. Microbiol. Biotechnol. 2008, 80, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Behera, M.; Jana, P.S.; More, T.T.; Ghangrekar, M. Rice mill wastewater treatment in microbial fuel cells fabricated using proton exchange membrane and earthen pot at different ph. Bioelectrochemistry 2010, 79, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhu, X.; Ni, J.; Borthwick, A. Palm oil mill effluent treatment using a two-stage microbial fuel cells system integrated with immobilized biological aerated filters. Bioresour. Technol. 2010, 101, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Kong, F.; Zheng, H.; Cao, D.; Ren, Y.; Yin, J. Electricity generation from synthetic penicillin wastewater in an air-cathode single chamber microbial fuel cell. Chem. Eng. J. 2011, 168, 572–576. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, R.; Liu, G.; Li, J.; Li, M.; Zhang, C. Electricity generation from indole and microbial community analysis in the microbial fuel cell. J. Hazard. Mater. 2010, 176, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, H.; Zhou, S.; Shi, C.; Wang, C.; Ni, J. A novel uasb–mfc–baf integrated system for high strength molasses wastewater treatment and bioelectricity generation. Bioresour. Technol. 2009, 100, 5687–5693. [Google Scholar] [CrossRef] [PubMed]
- Catal, T.; Bermek, H.; Liu, H. Removal of selenite from wastewater using microbial fuel cells. Biotechnol. Lett. 2009, 31, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ni, J. Simultaneous processes of electricity generation and p-nitrophenol degradation in a microbial fuel cell. Electrochem. Commun. 2009, 11, 274–277. [Google Scholar] [CrossRef]
- Luo, H.; Xu, P.; Roane, T.M.; Jenkins, P.E.; Ren, Z. Microbial desalination cells for improved performance in wastewater treatment, electricity production, and desalination. Bioresour. Technol. 2012, 105, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Mathuriya, A.S.; Sharma, V. Bioelectricity production from various wastewaters through microbial fuel cell technology. J. Biochem. Technol. 2010, 2, 133–137. [Google Scholar]
- Yu, C.-P.; Liang, Z.; Das, A.; Hu, Z. Nitrogen removal from wastewater using membrane aerated microbial fuel cell techniques. Water Res. 2011, 45, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Neburchilov, V.; Mehta, P.; Hussain, A.; Wang, H.; Guiot, S.; Tartakovsky, B. Microbial fuel cell operation on carbon monoxide: Cathode catalyst selection. Int. J. Hydrogen Energy 2011, 36, 11929–11935. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.; Boon, N.; Marzorati, M.; Verstraete, W. Enhanced removal of 1, 2-dichloroethane by anodophilic microbial consortia. Water Res. 2009, 43, 2936–2946. [Google Scholar] [CrossRef] [PubMed]
- Raghavulu, S.V.; Mohan, S.V.; Reddy, M.V.; Mohanakrishna, G.; Sarma, P. Behavior of single chambered mediatorless microbial fuel cell (mfc) at acidophilic, neutral and alkaline microenvironments during chemical wastewater treatment. Int. J. Hydrogen Energy 2009, 34, 7547–7554. [Google Scholar] [CrossRef]
- You, S.J.; Zhao, Q.L.; Jiang, J.Q.; Zhang, J.N.; Zhao, S.Q. Sustainable approach for leachate treatment: Electricity generation in microbial fuel cell. J. Environ. Sci. Health Part A 2006, 41, 2721–2734. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhang, X.; Li, Z.; Lei, L. Studies on treatment of chlorophenol-containing wastewater by microbial fuel cell. Chin. Sci. Bull. 2007, 52, 3448–3451. [Google Scholar] [CrossRef]
- Fu, L.; You, S.-J.; Zhang, G.-q.; Yang, F.-L.; Fang, X.-h. Degradation of azo dyes using in-situ fenton reaction incorporated into h 2 o 2-producing microbial fuel cell. Chem. Eng. J. 2010, 160, 164–169. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Haynes, R.J. Removal of pb (ii), cr (iii) and cr (vi) from aqueous solutions using alum-derived water treatment sludge. Water Air Soil Pollut. 2011, 215, 631–643. [Google Scholar] [CrossRef]
- Frontera-Suau, R.; Bost, F.D.; McDonald, T.J.; Morris, P.J. Aerobic biodegradation of hopanes and other biomarkers by crude oil-degrading enrichment cultures. Environ. Sci. Technol. 2002, 36, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Xu, Y.; Ye, Y.; Chen, Y.; Shen, S. Electricity generation from terephthalic acid using a microbial fuel cell. J. Chem. Technol. Biotechnol. 2009, 84, 356–360. [Google Scholar] [CrossRef]
- Morris, J.M.; Jin, S. Feasibility of using microbial fuel cell technology for bioremediation of hydrocarbons in groundwater. J. Environ. Sci. Health Part A 2007, 43, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wu, Y.; Zhao, L.; Sun, Q. Production of electricity from the treatment of continuous brewery wastewater using a microbial fuel cell. Fuel 2010, 89, 1381–1385. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, H.; Kim, H.; Kim, G.; Chang, I.; Lee, J.; Phung, N. Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl. Microbiol. Biotechnol. 2004, 63, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Ter Heijne, A.; Hamelers, H.V.; De Wilde, V.; Rozendal, R.A.; Buisman, C.J. A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5200–5205. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, Z.J. Bioelectrochemical metal recovery from wastewater: A review. Water Res. 2014, 66, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Damiano, L.; Jambeck, J.R.; Ringelberg, D.B. Municipal solid waste landfill leachate treatment and electricity production using microbial fuel cells. Appl. Biochem. Biotechnol. 2014, 173, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tanaka, Y.; Kuroda, K.; Hanajima, D.; Fukumoto, Y.; Yasuda, T.; Waki, M. Removal and recovery of phosphorous from swine wastewater by demonstration crystallization reactor and struvite accumulation device. Bioresour. Technol. 2007, 98, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Van Eerten-Jansen, M.C.; Jansen, N.C.; Plugge, C.M.; de Wilde, V.; Buisman, C.J.; ter Heijne, A. Analysis of the mechanisms of bioelectrochemical methane production by mixed cultures. J. Chem. Technol. Biotechnol. 2015, 90, 963–970. [Google Scholar] [CrossRef]
- Satar, I.; Daud, W.R.W.; Kim, B.H.; Somalu, M.R.; Ghasemi, M. Immobilized mixed-culture reactor (imcr) for hydrogen and methane production from glucose. Energy 2017, 139, 1188–1196. [Google Scholar] [CrossRef]
- Kumar, G.; Saratale, R.G.; Kadier, A.; Sivagurunathan, P.; Zhen, G.; Kim, S.-H.; Saratale, G.D. A review on bio-electrochemical systems (bess) for the syngas and value added biochemicals production. Chemosphere 2017, 177, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Rader, G.K.; Logan, B.E. Multi-electrode continuous flow microbial electrolysis cell for biogas production from acetate. Int. J. Hydrogen Energy 2010, 35, 8848–8854. [Google Scholar] [CrossRef]
- McAnulty, M.J.; Poosarla, V.G.; Kim, K.-Y.; Jasso-Chávez, R.; Logan, B.E.; Wood, T.K. Electricity from methane by reversing methanogenesis. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Kreysa, G.; Håkansson, B. Electrocatalysis by amorphous metals of hydrogen and oxygen evolution in alkaline solution. J. Electroanal. Chem. Interfacial Electrochem. 1986, 201, 61–83. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Guwy, A.; Dinsdale, R.; Kim, J.; Massanet-Nicolau, J.; Premier, G. Fermentative biohydrogen production systems integration. Bioresour. Technol. 2011, 102, 8534–8542. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.; Sleutels, T.H.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Jafary, T.; Daud, W.R.W.; Ghasemi, M.; Kim, B.H.; Carmona-Martínez, A.A.; Bakar, M.H.A.; Jahim, J.M.; Ismail, M. A comprehensive study on development of a biocathode for cleaner production of hydrogen in a microbial electrolysis cell. J. Clean. Prod. 2017, 164, 1135–1144. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, E.; Zhang, J.; Dai, Y.; Yang, Z.; Christensen, H.E.; Ulstrup, J.; Zhao, F. Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Long-term operation of microbial electrosynthesis systems improves acetate production by autotrophic microbiomes. Environ. Sci. Technol. 2013, 47, 6023–6029. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Arends, J.B.; Vanwonterghem, I.; Van Meerbergen, J.; Guo, K.; Tyson, G.W.; Rabaey, K. Selective enrichment establishes a stable performing community for microbial electrosynthesis of acetate from CO2. Environ. Sci. Technol. 2015, 49, 8833–8843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escapa, A.; Mateos, R.; Martínez, E.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Tang, H.; Zeng, Y.; Zeng, Y.; Wang, R.; Cai, S.; Liao, C.; Cai, H.; Lu, X.; Tsiakaras, P. Iron-embedded nitrogen doped carbon frameworks as robust catalyst for oxygen reduction reaction in microbial fuel cells. Appl. Catal. B Environ. 2017, 202, 550–556. [Google Scholar] [CrossRef]
- Thostenson, J.O.; Ngaboyamahina, E.; Sellgren, K.L.; Hawkins, B.T.; Piascik, J.R.; Klem, E.J.; Parker, C.B.; Deshusses, M.A.; Stoner, B.R.; Glass, J.T. Enhanced H2O2 production at reductive potentials from oxidized boron-doped ultrananocrystalline diamond electrodes. ACS Appl. Mater. Interfaces 2017, 9, 16610–16619. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; You, S.J.; Yang, F.l.; Gao, M.m.; Fang, X.h.; Zhang, G.Q. Synthesis of hydrogen peroxide in microbial fuel cell. J. Chem. Technol. Biotechnol. 2010, 85, 715–719. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Ali, N.; Jamil, S.U.U.; Waseem, H.; Khan, K.; Pan, G. Insight into eco-friendly fabrication of silver nanoparticles by pseudomonas aeruginosa and its potential impacts. J. Environ. Chem. Eng. 2017, 5, 3266–3272. [Google Scholar] [CrossRef]
- Ali, J.; Hameed, A.; Ahmed, S.; Ali, M.I.; Zainab, S.; Ali, N. Role of catalytic protein and stabilising agents in the transformation of ag ions to nanoparticles by pseudomonas aeruginosa. IET Nanobiotechnol. 2016, 10, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Commault, A.S.; Laczka, O.; Siboni, N.; Tamburic, B.; Crosswell, J.R.; Seymour, J.R.; Ralph, P.J. Electricity and biomass production in a bacteria-chlorella based microbial fuel cell treating wastewater. J. Power Sources 2017, 356, 299–309. [Google Scholar] [CrossRef]
- Saba, B.; Christy, A.D.; Yu, Z.; Co, A.C. Sustainable power generation from bacterio-algal microbial fuel cells (mfcs): An overview. Renew. Sustain. Energy Rev. 2017, 73, 75–84. [Google Scholar] [CrossRef]
- Xie, T.; Reddy, K.R.; Wang, C.; Yargicoglu, E.; Spokas, K. Characteristics and applications of biochar for environmental remediation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Lawton, R.J.; Cole, A.J.; Roberts, D.A.; Paul, N.A.; de Nys, R. The industrial ecology of freshwater macroalgae for biomass applications. Algal. Res. 2017, 24, 486–491. [Google Scholar] [CrossRef]
| Name of Bacteria | Electron Transfer Mechanism | Molecules Involved in Electron Transfer | Remarks | References |
|---|---|---|---|---|
| Geobacter sulfurreducens | DET (nanowires) | Branched OMCs system: cytochromes (c-, d-types) and Type IV pili | Oxidation of organic compounds to iron reduction leads to electron release | [35] |
| Thermincola ferriacetica | DET | c-type cytochromes | Electrons are transported to electrode through multiheme c-type cytochromes | [36] |
| Clostridium ljungdahlii | DET | Rnf complex (Ferredoxin:NAD+ -oxidoreductase) (No cytochromes, no quinones) | Electron bifurcating ferredoxin reduction | [37,38] |
| Pseudomonas aeruginosa | DET | cytochromes (a-, b-, c-, o-type), phenazines, flavines (soluble and bound), quinones and dehydrogenases | Electron transfer through the production of versatile phenazine redox mediators. | [39] |
| Geobacter metallireducens | DET | c-Type cytochromes, that is, OmcB and OmcE | Fe(III) oxide reduction | [40] |
| Shewanella putrefaciens | DET | c-Type cytochromes, that is, MtrC and OmcA, FAD transporter | NIF | [41] |
| Rhodopseudomonas Palustris | DET | c-Type cytochromes | NIF | [42] |
| Klebsiella pneumoniae | DET | 2,6-di-tert-butyl-p-benzoquinone | Reduction of iron oxide and generate electricity | [43] |
| Moorella thermoacetica | IET (methylviologen) | Cytochromes (b, d-type), quinones and/or Ech-complex | Reduction of CO2 to other organic compounds | [37] |
| Acetobacterium woodii | IET (biotic H2) | Rnf complex (Ferredoxin: NAD+ -fuctase), membrane bound corrinoids (No cytochromes, no quinones) | Electron bifurcating ferredoxin hydrogenase catalysis H2 formation | [37,44] |
| Lactococcus lactis | IET | 2-Amino-3-dicarboxy-1,4-naphthoquinone | NIF | [45] |
| Shewanella oneidensis | IET (self-produced shuttles),DET (nanowires) | Metal-reducing pathway components CymA, MtrA, MtrB, MtrC, and OmcA | CymA in inner-membrane oxidizes the quinol and transfers the released electrons to MtrA directly or indirectly | [46] |
| Sporomusa ovata | DET and IET | Membrane-bound cytochromes (b-, c-types) and quinones | CO2 is used as an electron acceptor and reduced to organic compounds via the Wood-Ljungdahl pathway | [37] |
| Shewanella oneidensis | IET (self-produced shuttles), DET (nanowires) | Flavins, riboflavin | NIF | [47] |
| Thermincola ferriacetica | NIF | Anthraquinone-2,6-disulfonate | Fe(III) reduction | [36] |
| Isolated Electroactive Bacteria | Source of Isolation | Technique Used before Culturing | Reference |
|---|---|---|---|
| Dietzia sp. RNV-4 | Single Chamber MFC | Dilution | [53] |
| Geobacter OS1 | Petroleum hydrocarbon-contaminated soil | Dilution | [54] |
| Ochrobactrum OS2 | Petroleum hydrocarbon-contaminated soil | Dilution | [54] |
| Ochrobactrum anthropi YZ-1 | Single Chamber MFC | Dilution & Enrichment | [55] |
| Sphingomonas strain DJ | Microbial electrolysis cell (MECs) | Dilution | [56] |
| Rhodopseudomonas palustris DX-1 | Air cathode MFC | Dilution | [57] |
| Geobacter SD-1 | Microbial electrolysis cell (MECs) | Dilution & Enrichment | [58] |
| Citrobacter LAR-1 | Sediments | Dilution & Enrichment | [59] |
| Geobacter bremensis | Compost | Dilution & Enrichment | [60] |
| Bacillus sediminis DX-5T | Microbial electrolysis cell (MECs) | Dilution | [61] |
| Clostridium butyricum | Mediatorless MFC | Enrichment | [62] |
| Rhodoferax ferrireducens | Sediments | Enrichment | [63] |
| Enterococcus gallinarum MG25 | Submerged soil | Dilution & Enrichment | [64] |
| Aeromonas hydrophila | Mediatorless MFC | Enrichment | [65] |
| Type of Wastewater Treated | Inoculum Source | Hydraulic Retention Time | Initial COD | COD Removal Efficiency | Power Density | Reference |
|---|---|---|---|---|---|---|
| Cereal wastewater | Sludge | 120 h | 595 mg/L | 95% | 371 ± 10 mW/m2 | [101] |
| Dairy wastewater | Anaerobic mixed consortia as | NIF | 4.44 kgCOD/m3 | 95.49% | 1.10 W/m3 | [102] |
| Dioscorea zingiberensis wastewater | Anaerobic sludge | 72 h | 91 000 mg/L | 93.50% | 118.1 mW/m2 | [103] |
| Effluent from hydrogen producing biofermentor | Domestic wastewater | 23 h | 6.3 g/L | 97% | 4200 mW/m3 | [104] |
| Confectionary wastewater | Aerobic sludge, anaerobic sludge, | NIF | 22 000 mg/L | 92% | NIF | [105] |
| Biodiesel waste | Domestic wastewater | NIF | 1400 mg/L | 90% | 1310 ± 15 mW/m2 | [106] |
| Starch processing wastewater | Wastewater itself | four cycles of 140 days | 4852 mg/L | 98% | 239.4 mW/m2 | [107] |
| Paper wastewater | Wastewater itself | 500 h | 0.48 g/L | 76 ± 4% TCOD; | 501 ± 20 mW/m2 | [108] |
| Rice mill wastewater | Anaerobic sludge | 288 h | 1100–1125 mg/L | 96.5% COD | 2.3 W/m3 | [109] |
| Palm oil effluent | 3.5 g/L sludge | 48 h | 10000 mg/L | COD; 93.6% | NIF | [110] |
| Synthetic penicillin wastewater | Bacteria from another MFC | 24 h | 50 mg/L penicillin: 1000 mg/L glucose mix | 98% | 101.2 mW/m3 | [111] |
| Indole | Mixed aerobic and anaerobic activated | 12 h | 500 mg/L | Complete removal | 1410.2 mW/m2 | [112] |
| Quinoline | – | 6 h | 500 mg/L | Complete removal | 16.4 mW/m2 | [113] |
| Selenite wastewater | Mixed bacterial culture | 48 and 72 h | 50 and 200 mg/L | 99% | 2900 mW/m2 | [114] |
| Pyridine | Mixed aerobic and anaerobic activated | 12 h | 500 mg/L | Complete removal | 1410.2 mW/m2 | [114] |
| Ceftriaxone sodium (Cs). | Bacteria from another glucose-fed mFC | 24 h | 50 mg/L (Cs): 1000 mg/L glucose | 96% | 11 mW/m2 | [111] |
| p-Nitrophenol wastewater | Anaerobic sludge | 12 h | NIF | Complete degradation | 143 mW/m2 | [115] |
| Refractory contaminants (Furfural) | Anaerobic and aerobic sludge | 60 h | 300 mg/L | 96% COD; 100% furfural | 15.9 W/m3 | [116] |
| Dairy wastewater | self-microbial population of | 10 days | 1487 mg/L | 81.29% | 10.89 mA | [117] |
| Carbonaceous and nitrogenous pollutants | digested sludge | 210 days | 545 ± 43 mg/L | 99% COD; <20% | by 50% 0.25 ± 0.07 V | [118] |
| CO | anaerobic sludge | 14 days | NIF | NIF | 260.3 mV | [119] |
| 1,2-Dichloro-ethane waste water | mixed natural consortium from a 1,2-DCA contaminated site | 1 month | 99 mg/L | 85% | 0.03 mA | [120] |
| Chemical wastewater | anaerobic mixed consortia | 96 h | 5900 mg/L | 58.98% | 186.34 mA/m2 | [121] |
| Landfill | anaerobic | NIF | 9800 mg/L | NIF | 6817.4 mW/m3 | [122] |
| 4-Chlorophenol | anaerobic sludge | 45 h | 60 mg/L | complete | 12.4 mW/m2 | [123] |
| Amaranth Dye | NIF | 1 h | 75 mg/L | 82.59% | 28.3W/m3 | [124] |
| Cr(VI) | NIF | 10 h | 177 mg/L | 92.80% | 108 mW/m2 | [125] |
| Refinery waste | NIF | 6 days | NIF | NIF | 120 mW/m2 | [126] |
| Terephthalic acid | anaerobic sludge | 210 h | 4000 mg/L | 80.30% | 96.3 mW/m2 | [127] |
| Diesel range organics | diesel contaminated groundwater | 21 days | 300 mg/L | 82% | 31 mW/m2 | [128] |
| Brewery wastewater | anaerobic mixed consortia | 2.13 h | 1501 mg/L | 47.60% | 669 mW/m2 | [129] |
| Starch processing wastewater | electrochemically active bacteria | 6 weeks | 1700 mg/L | 97% | 0.44 mA/cm2 | [130] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, J.; Sohail, A.; Wang, L.; Rizwan Haider, M.; Mulk, S.; Pan, G. Electro-Microbiology as a Promising Approach Towards Renewable Energy and Environmental Sustainability. Energies 2018, 11, 1822. https://doi.org/10.3390/en11071822
Ali J, Sohail A, Wang L, Rizwan Haider M, Mulk S, Pan G. Electro-Microbiology as a Promising Approach Towards Renewable Energy and Environmental Sustainability. Energies. 2018; 11(7):1822. https://doi.org/10.3390/en11071822
Chicago/Turabian StyleAli, Jafar, Aaqib Sohail, Lei Wang, Muhammad Rizwan Haider, Shahi Mulk, and Gang Pan. 2018. "Electro-Microbiology as a Promising Approach Towards Renewable Energy and Environmental Sustainability" Energies 11, no. 7: 1822. https://doi.org/10.3390/en11071822