Performance and Emission Analysis of Rubber Seed, Palm, and Their Combined Blend in a Multi-Cylinder Diesel Engine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biodiesel Production
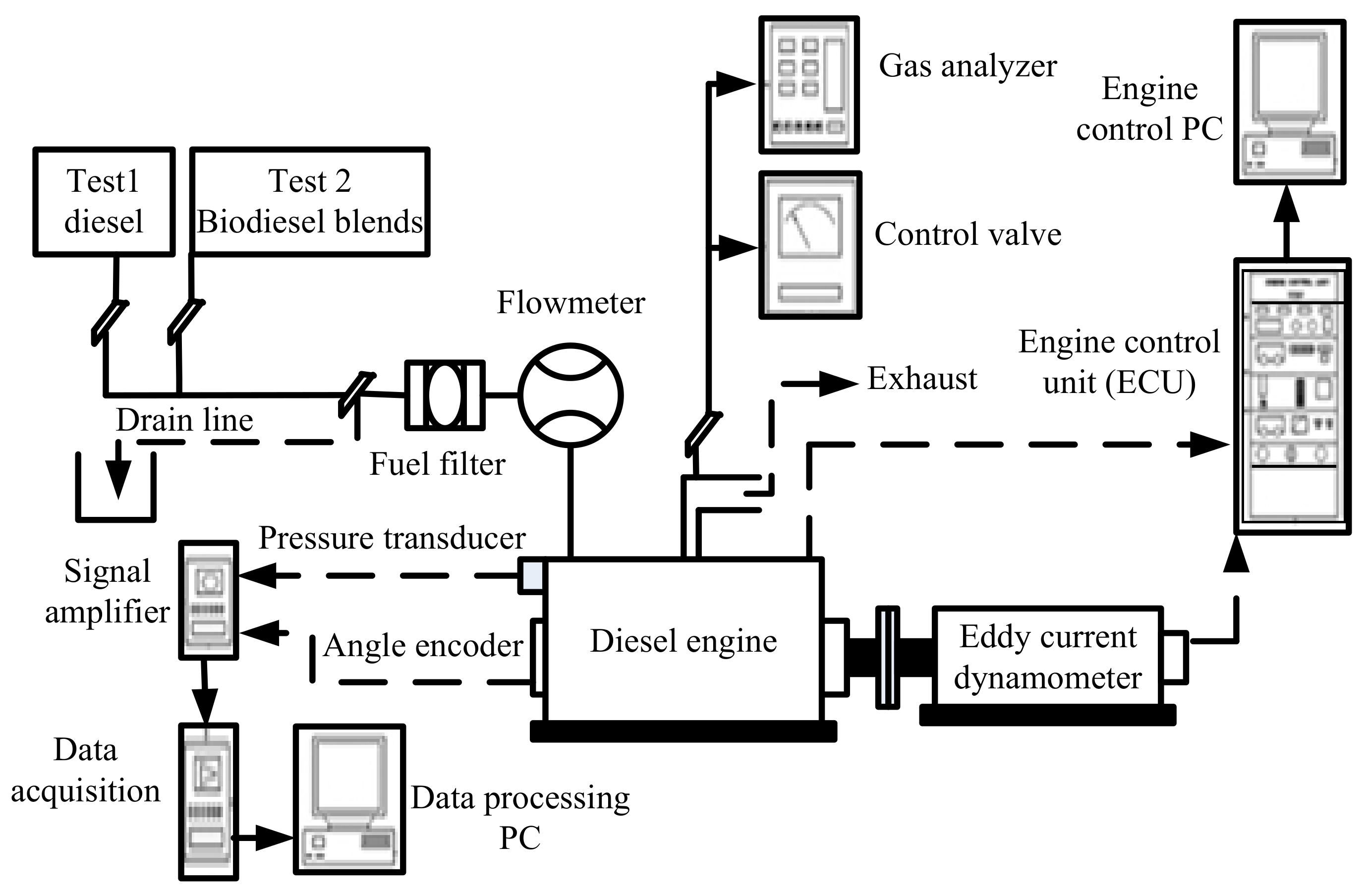
2.3. Experimental Set-Up
2.4. Uncertainty Analysis
3. Results and Discussion
3.1. Analysis of Biodiesel Fuel Properties
3.2. Performance Analysis
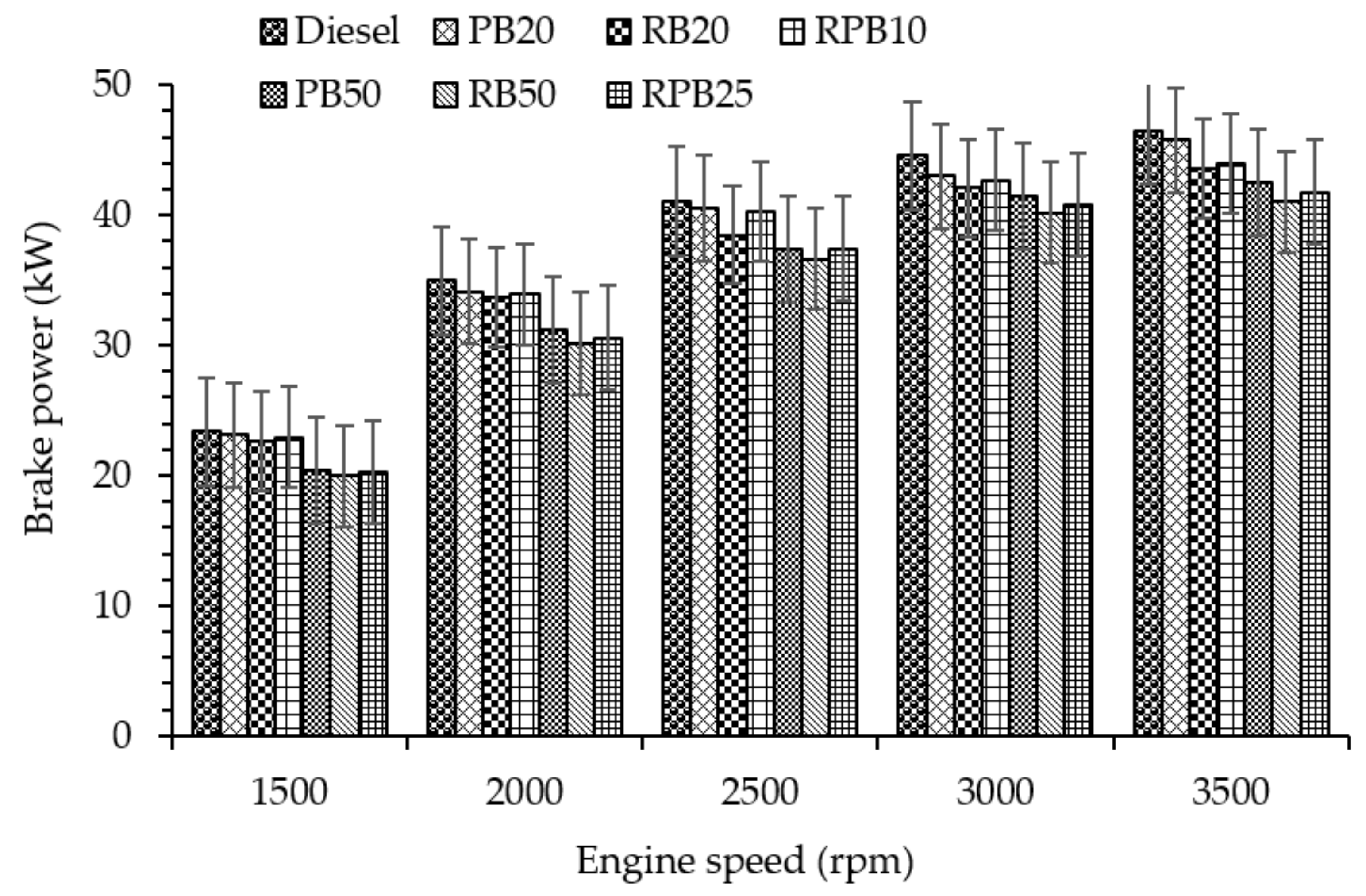
3.2.1. Brake Power (BP)
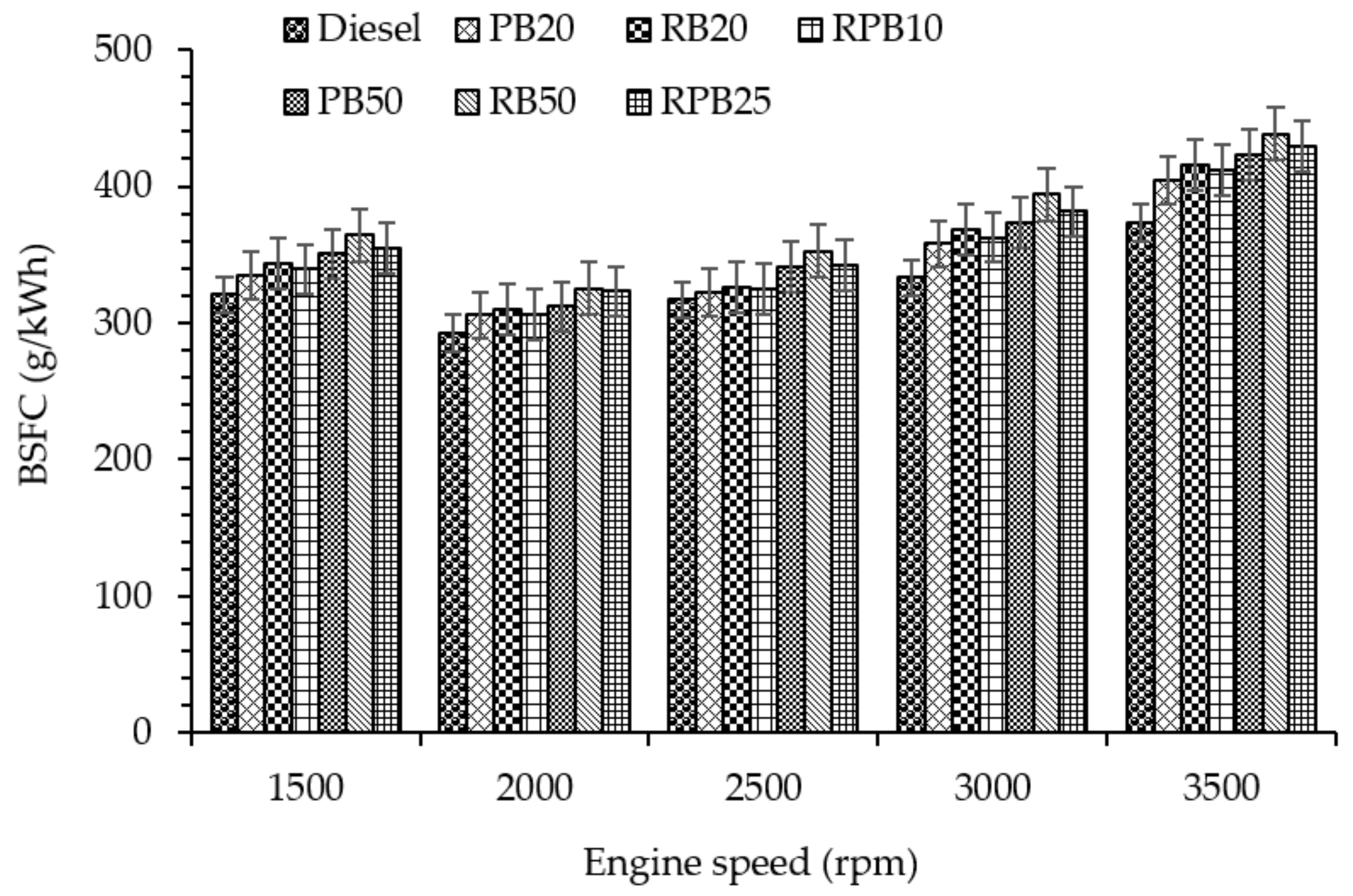
3.2.2. Brake Specific Fuel Consumption (BSFC)
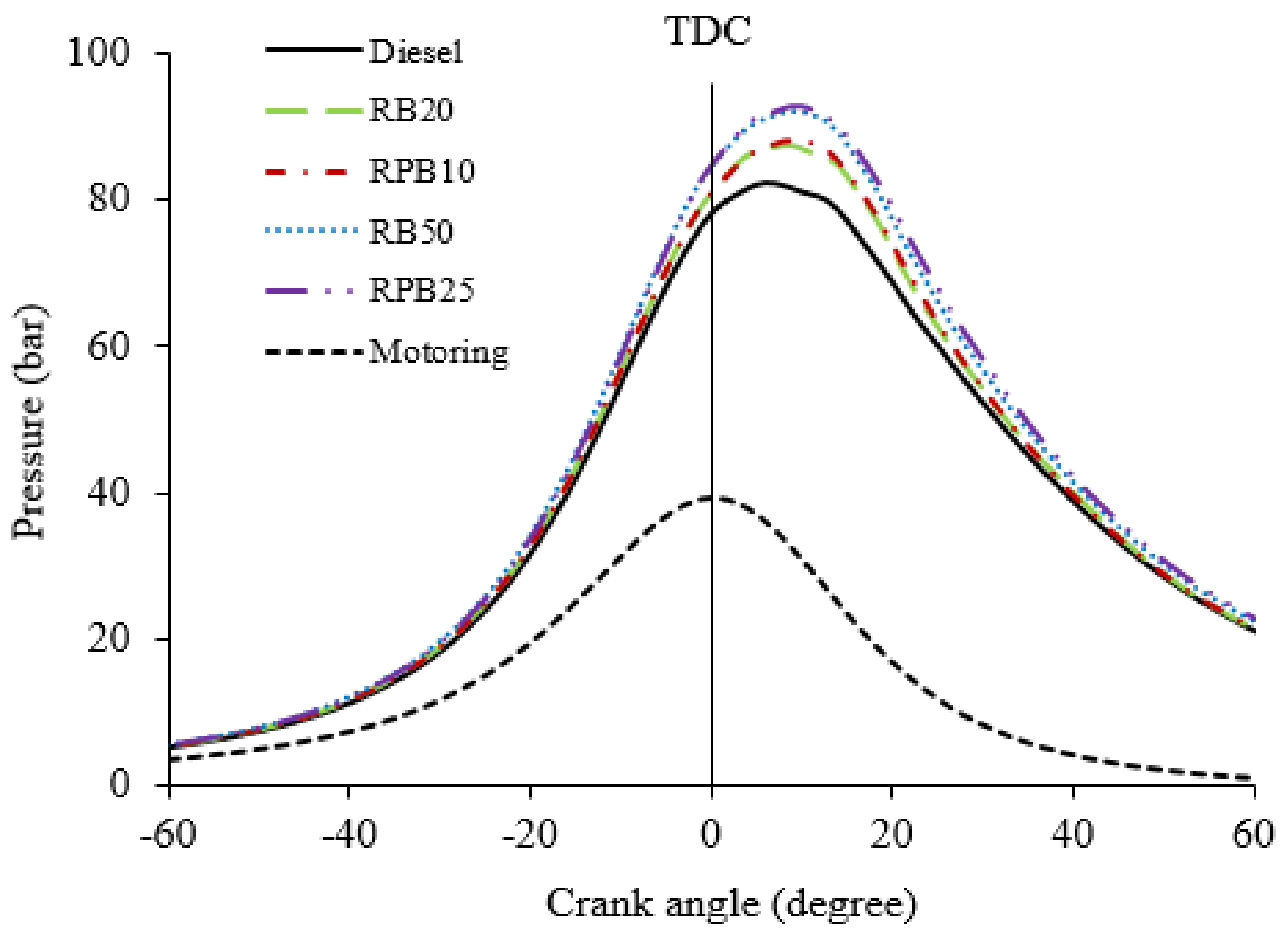
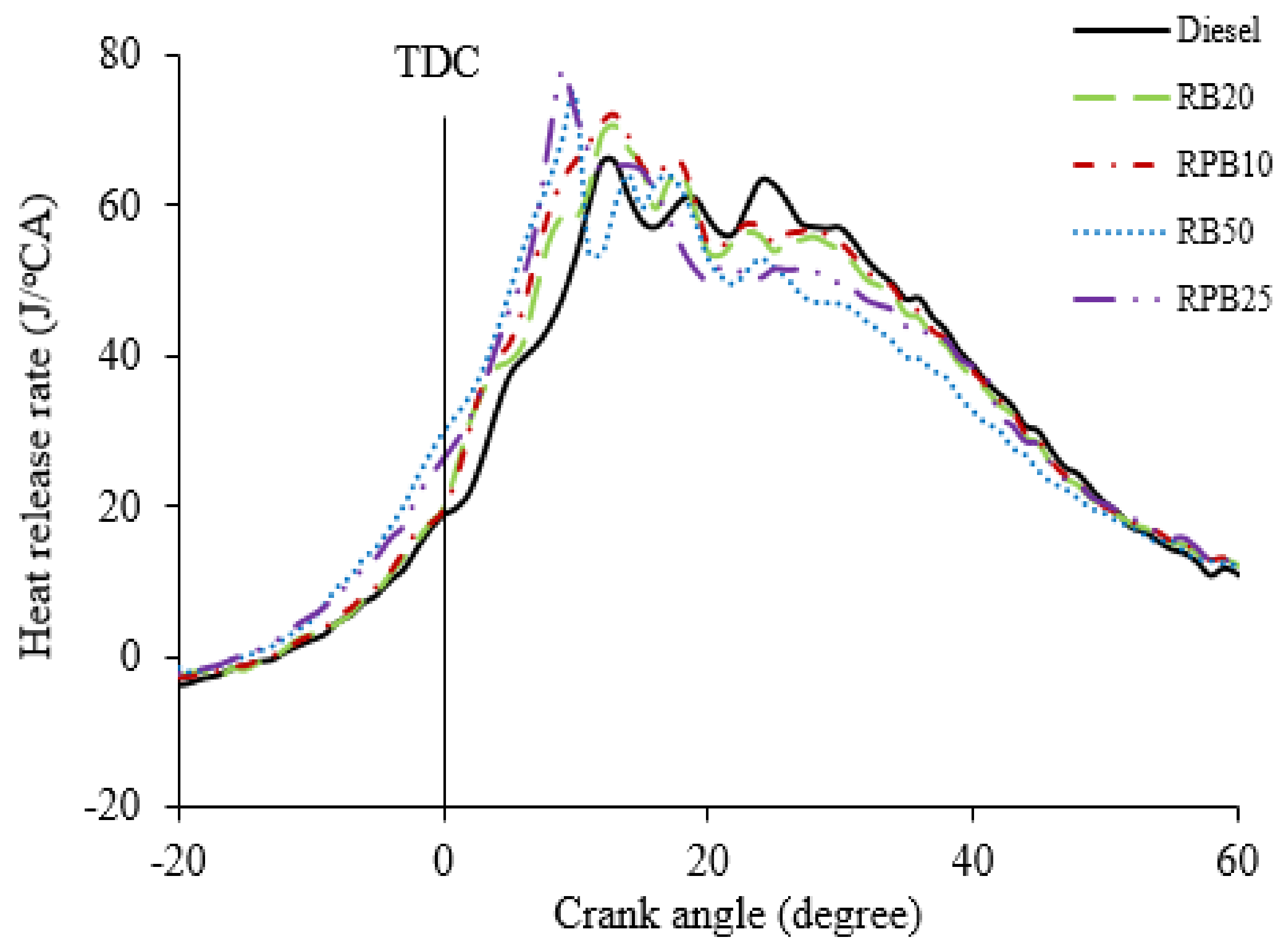
3.3. Combustion Analysis
3.4. Emissions Analysis
3.4.1. Carbon Monoxide (CO) Emission
3.4.2. Hydrocarbon Emission (HC)
3.4.3. Carbon Dioxide (CO2) Emission
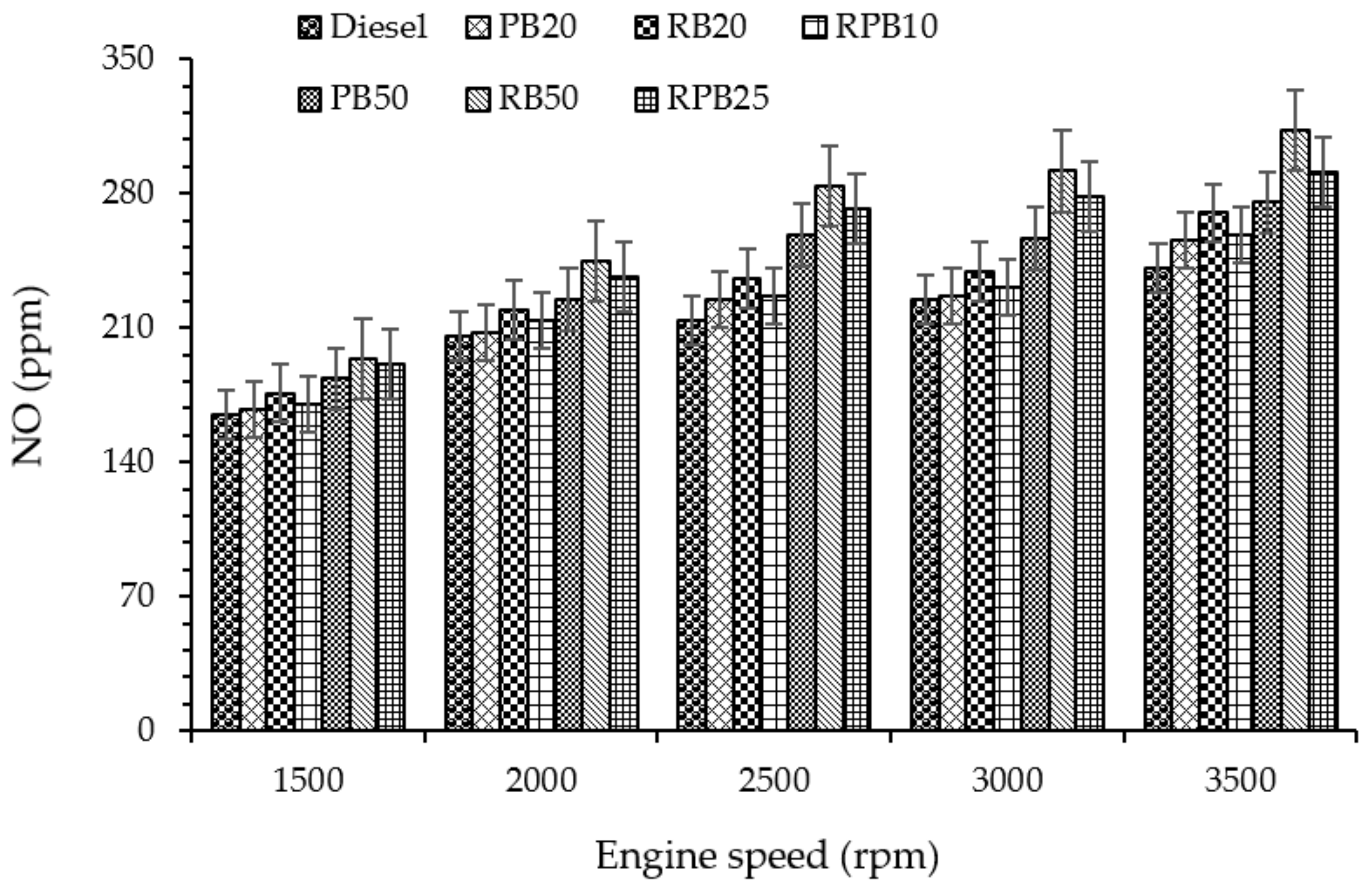
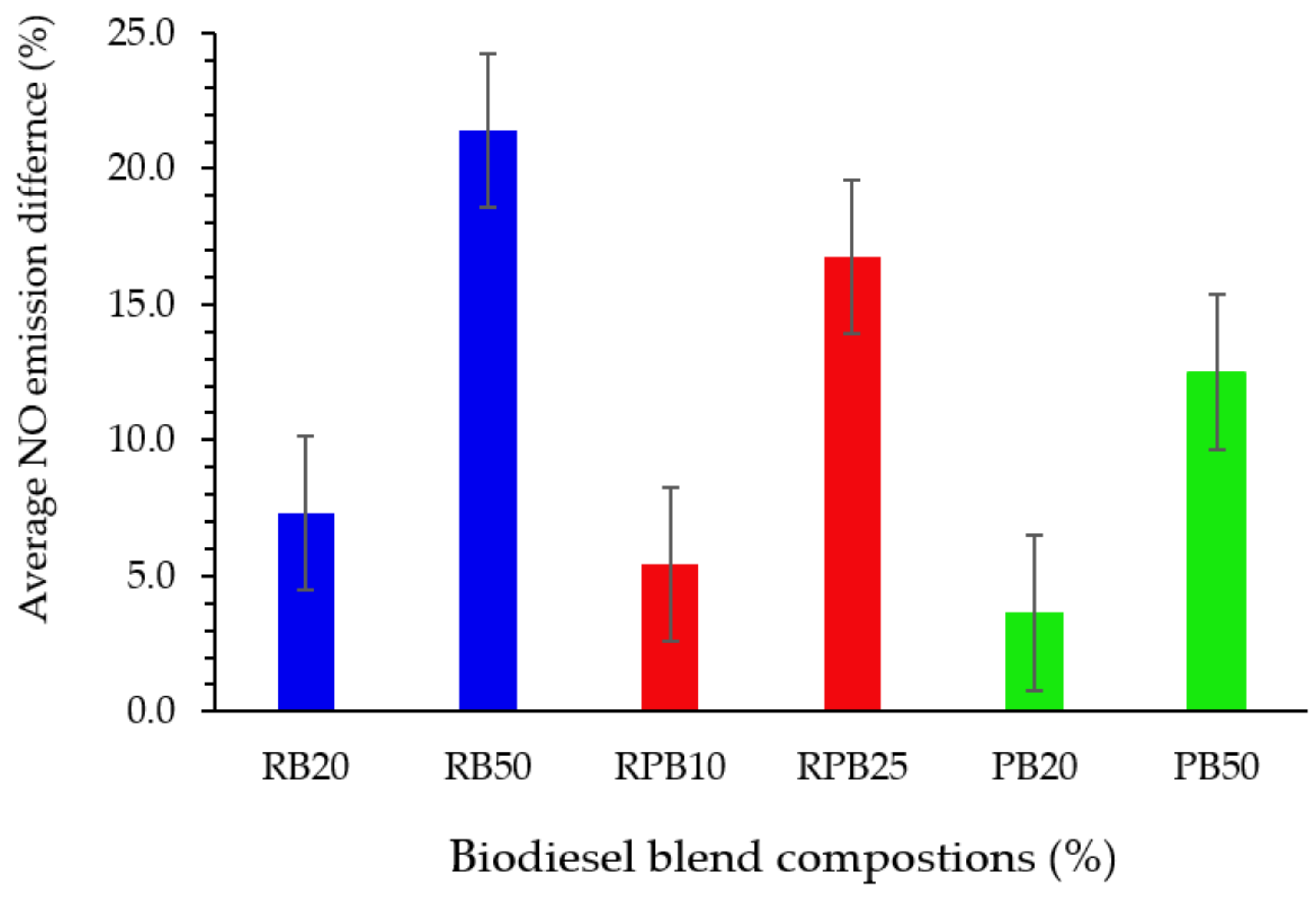
3.4.4. Nitric Oxide (NO) Emission
3.4.5. Smoke Opacity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RSO | Rubber seed oil |
| RB | Rubber seed biodiesel |
| PB | Palm oil biodiesel |
| RPB | Rubber seed-palm oil biodiesel |
| BP | Brake power |
| BSFC | Brake specific fuel consumption |
| CO | Carbon monoxide |
| HC | Hydrocarbon |
| HRR | Heat release rate |
| SOC | Start of combustion |
| BTDC | Before top dead left |
| JB | Jatropha biodiesel |
| PB10 | 10% palm oil biodiesel + 90% diesel fuel |
| JB10 | 10% jatropha biodiesel + 90% diesel fuel |
| PBJB5 | 5% palm oil biodiesel + 5% jatropha biodiesel + 90% diesel |
| PB20 | 20% palm oil biodiesel + 80% diesel fuel |
| JB20 | 20% jatropha biodiesel + 80% diesel fuel |
| PBJB10 | 10% palm oil biodiesel + 10% jatropha biodiesel + 80% diesel fuel |
| PB30 | 30% palm oil biodiesel + 70% diesel fuel |
| CB | Coconut biodiesel |
| CB30 | 30% coconut biodiesel + 70% diesel fuel |
| PB15CB15 | 15% palm oil biodiesel + 15% coconut biodiesel + 70% diesel fuel |
| PC | Palm-coconut biodiesel |
| JPC | Jatropha-palm-coconut biodiesel |
| CPO | Crude palm oil |
| RSE | Relative standard error |
References
- Mofijur, H.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E.; Fattah, I.M.R.; Mobarak, H.M. Comparative evaluation of performance and emission characteristics of Moringa oleifera and Palm oil based biodiesel in a diesel engine. Ind. Crops Prod. 2014, 53, 78–84. [Google Scholar] [CrossRef]
- Fattah, I.R.; Masjuki, H.; Liaquat, A.; Ramli, R.; Kalam, M.; Riazuddin, V. Impact of various biodiesel fuels obtained from edible and non-edible oils on engine exhaust gas and noise emissions. Renew. Sustain. Energy Rev. 2013, 18, 552–567. [Google Scholar] [CrossRef]
- Piemonte, V.; di Paola, L.; Iaquaniello, G.; Prisciandaro, M. Biodiesel production from microalgae: Ionic liquid process simulation. J. Clean. Prod. 2016, 111, 62–68. [Google Scholar] [CrossRef]
- Malaysian Oil Palm Statistics 2007; Economics and Industry Development Division, MPOB: Selangor, Malaysia, 2008.
- Biofuels Annual Kuala Lumpur Malaysia. 2016. Available online: https://gain.fas.usda.gov (accessed on 29 March 2018).
- Wendy, N.; Qin, P.; Loong, L.H.; Yusup, S. Supply network synthesis on rubber seed oil utilisation as potential biofuel feedstock. Energy 2013, 55, 82–88. [Google Scholar]
- Lim, W.H.; Ooi, T.L.; Hong, H.K. Study on low temperature properties of palm oil methyl esters-petrodiesel blends. J. Oil Palm Res. 2009, 21, 683–692. [Google Scholar]
- Lam, M.K.; Tan, K.T.; Lee, K.T.; Mohamed, A.R. Malaysian palm oil: Surviving the food versus fuel dispute for a sustainable future. Renew. Sustain. Energy Rev. 2009, 13, 1456–1464. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Abdullah, A.Z.; Salamatinia, B.; Mootabadi, H.; Bhatia, S. Current status and policies on biodiesel industry in Malaysia as the world’s leading producer of palm oil. Energy Policy 2009, 37, 5440–5448. [Google Scholar] [CrossRef]
- Eka, H.D.; Aris, T.Y.; Nadiah, W.W. Potential use of Malaysian rubber (Hevea brasiliensis) seed as food, feed and biofue. Int. Food Res. J. 2010, 17, 527–534. [Google Scholar]
- Abdullah, B.M.; Salimon, J. Physicochemical characteristics of Malaysian rubber (Hevea brasiliensis) seed oil. Eur. J. Sci. Res. 2009, 31, 437–445. [Google Scholar]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Fattah, I.M.R.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Knothe, G. Designer biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Jena, R.H.; Jadav, P.C.; Snehal, S. Performance of a diesel engine with blends of biodiesel (from a mixture of oils) and high-speed diesel. Int. J. Energy Environ. Eng. 2013, 4, 1. [Google Scholar]
- Haas, M.J.; Scott, K.M.; Alleman, T.L.; McCormick, R.L. Engine Performance of Biodiesel Fuel Prepared from Soybean Soapstock: A High Quality Renewable Fuel Produced from a Waste Feedstock. Energy Fuels 2001, 15, 1207–1212. [Google Scholar] [CrossRef]
- Can, Ö. Combustion characteristics, performance and exhaust emissions of a diesel engine fueled with a waste cooking oil biodiesel mixture. Energy Convers. Manag. 2014, 87, 676–686. [Google Scholar] [CrossRef]
- Öztürk, E. Performance, emissions, combustion and injection characteristics of a diesel engine fuelled with canola oil–hazelnut soapstock biodiesel mixture. Fuel Process. Technol. 2015, 129, 183–191. [Google Scholar] [CrossRef]
- Sanjid, A.; Hassan, M.H.; Kalam, M.A.; Rahman, A.S.M.; Abedin, M.J.; Palash, S.M. Production of palm and jatropha based biodiesel and investigation of palm-jatropha combined blend properties, performance, exhaust emission and noise in an unmodified diesel engine. J. Clean. Prod. 2014, 65, 295–303. [Google Scholar] [CrossRef]
- Habibullah, M.; Masjuki, H.H.; Kalam, M.A.; Fattah, I.M.R.; Ashraful, A.M.; Mobarak, H.M. Biodiesel production and performance evaluation of coconut, palm and their combined blend with diesel in a single-cylinder diesel engine. Energy Convers. Manag. 2014, 87, 250–257. [Google Scholar] [CrossRef]
- Habibullah, M.S.; Fattah, I.M.R.; Masjuki, H.H.; Kalam, M.A. Effects of Palm–Coconut biodiesel blends on the performance and emission of a single-cylinder diesel engine. Energy Fuels 2015, 29, 734–743. [Google Scholar] [CrossRef]
- Arbab, M.I.; Varman, M.; Masjuki, H.H.; Kalam, M.A.; Imtenan, S.; Sajjad, H.; Rizwanul Fattah, I.M. Evaluation of combustion, performance, and emissions of optimum palm–coconut blend in turbocharged and non-turbocharged conditions of a diesel engine. Energy Convers. Manag. 2015, 90, 111–120. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Varman, M.; Hassan, M.H.; Kalam, M.A.; Hossain, S.; Sayeed, I. Tailoring fuel properties using jatropha, palm and coconut biodiesel to improve CI engine performance and emission characteristics. J. Clean. Prod. 2015, 101, 262–270. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Das, L.M. Combustion analysis of Jatropha, Karanja and Polanga based biodiesel as fuel in a diesel engine. Fuel 2009, 88, 994–999. [Google Scholar] [CrossRef]
- Firestone, D. Official Methods and Recommended Practices of the AOCS; American Oil Chemists’ Society: Urbana, IL, USA, 2009. [Google Scholar]
- Anton, P. Instruction Manual: DMA 4100 M, DMA 4500 M, DMA 5000 M; Anton Paar: Graz, Austria, 2012. [Google Scholar]
- Chuah, L.F.; Yusup, S.; Aziz, A.R.A.; Bokhari, A.; Abdullah, M.Z. Cleaner production of methyl ester using waste cooking oil derived from palm olein using a hydrodynamic cavitation reactor. J. Clean. Prod. 2015, 112, 4505–4514. [Google Scholar] [CrossRef]
- Canakci, M. Combustion characteristics of a turbocharged DI compression ignition engine fueled with petroleum diesel fuels and biodiesel. Bioresour. Technol. 2007, 98, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.; Chuah, L.F.; Yusup, S.; Klemeš, J.J.; Kamil, R.N.M. Optimisation on pretreatment of rubber seed (Hevea brasiliensis) oil via esterification reaction in a hydrodynamic cavitation reactor. Bioresour. Technol. 2015, 199, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.; Chuah, L.F.; Yusup, S.; Klemeš, J.J.; Akbar, M.M.; Kamil, R.N.M. Cleaner production of rubber seed oil methyl ester using a hydrodynamic cavitation: Optimisation and parametric study. J. Clean. Prod. 2016, 136, 31–41. [Google Scholar] [CrossRef]
- Ahmad, J.; Yusup, S.; Bokhari, A.; Nik Mohammad Kamil, R. Study of fuel properties of rubber seed oil based biodiesel. Energy Convers. Manag. 2014, 78, 266–275. [Google Scholar] [CrossRef]
- Yusri, I.; Mamat, R.; Azmi, W.; Omar, A.; Obed, M.; Shaiful, A. Application of response surface methodology in optimization of performance and exhaust emissions of secondary butyl alcohol-gasoline blends in SI engine. Energy Convers. Manag. 2017, 133, 178–195. [Google Scholar] [CrossRef]
- ASTM. Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels; ASTM D6751; ATSM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- CEN. Automotive Fuels-Fatty Acid Methyl Esters (FAME) for Diesel Engines-Requirements and Test Methods; EN 14214; European Committee for Standardization: Brussels, Belgium, 2008. [Google Scholar]
- Thaiyasuit, K.P.P.; Milton, B. Combustion efficiency and performance of RSO biodiesel as alternative fuel in a single cylinder CI engine. Energy Explor. Exploit. 2012, 30, 153–166. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Kumar, N.; Cho, H.M. A study on the performance and emission of a diesel engine fueled with Jatropha biodiesel oil and its blends. Energy 2012, 37, 616–622. [Google Scholar] [CrossRef]
- Buyukkaya, E.; Benli, S.; Karaaslan, S.; Guru, M. Effects of trout-oil methyl ester on a diesel engine performance and emission characteristics. Energy Convers. Manag. 2013, 69, 41–48. [Google Scholar] [CrossRef]
- Goodrum, J. Volatility and boiling points of biodiesel from vegetable oils and tallow. Biomass Bioenergy 2002, 22, 205–211. [Google Scholar] [CrossRef]
- Qi, D.; Chen, H.; Geng, L.; Bian, Y.Z. Experimental studies on the combustion characteristics and performance of a direct injection engine fueled with biodiesel/diesel blends. Energy Convers. Manag. 2010, 51, 2985–2992. [Google Scholar] [CrossRef]
- Canakci, M.; Ozsezen, A.N.; Arcaklioglu, E.; Erdil, A. Prediction of performance and exhaust emissions of a diesel engine fueled with biodiesel produced from waste frying palm oil. Expert Syst. Appl. 2009, 36, 9268–9280. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chonga, W.T. Experimental study on performance and exhaust emissions of a diesel engine fuelled with Ceiba pentandra biodiesel blends. Energy Convers. Manag. 2013, 76, 828–836. [Google Scholar] [CrossRef]
- Ong, H.H.M.H.C.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Leong, K.Y. Optimization of biodiesel production and engine performance from high free fatty acid Calophyllum inophyllum oil in CI diesel engine. Energy Convers. Manag. 2014, 81, 30–40. [Google Scholar] [CrossRef]
- Özener, O.; Yüksek, L.; Ergenç, A.T.; Özkan, M. Effects of soybean biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel 2014, 115, 875–883. [Google Scholar] [CrossRef]
- Sanjid, A.; Kalam, M.A.; Masjuki, H.H.; Varman, M.; Zulkifli Nurin Wahidah, B.M.; Abedin, M.J. Performance and emission of multi-cylinder diesel engine using biodiesel blends obtained from mixed inedible feedstocks. J. Clean. Prod. 2016, 112, 4114–4122. [Google Scholar] [CrossRef]
- Yasin, M.M.; Yusaf, T.; Mamat, R.; Yusop, A.F. Characterization of a diesel engine operating with a small proportion of methanol as a fuel additive in biodiesel blend. Appl. Energy 2014, 114, 865–873. [Google Scholar] [CrossRef]
- Teoh, Y.; Masjuki, H.; Kalam, M.; Amalina, M.; How, H. Impact of Waste Cooking Oil Biodiesel on Performance, Exhaust Emission and Combustion Characteristics in a Light-Duty Diesel Engine; SAE Technical Paper 0148-7191; SAE: Warrendale, PA, USA, 2013. [Google Scholar]
- Muralidharan, K.; Vasudevan, D.; Sheeba, K. Performance, emission and combustion characteristics of biodiesel fuelled variable compression ratio engine. Energy 2011, 36, 5385–5393. [Google Scholar] [CrossRef]
- İleri, E.; Koçar, G. Effects of antioxidant additives on engine performance and exhaust emissions of a diesel engine fueled with canola oil methyl ester–diesel blend. Energy Convers. Manag. 2013, 76, 145–154. [Google Scholar] [CrossRef]
- Gumus, M.; Sayin, C.; Canakci, M. The impact of fuel injection pressure on the exhaust emissions of a direct injection diesel engine fueled with biodiesel-diesel fuel blends. Fuel 2012, 95, 486–494. [Google Scholar] [CrossRef]
- Singh, M.; Sandhu, S.S. Performance and emission characteristics of an indirect injection (IDI) multi-cylinder compression ignition (CI) engine using diesel/Argemone maxicana biodiesel blends. RSC Adv. 2015, 5, 91069–91081. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Fattah, I.R.; Masjuki, H.; Kalam, M.; Wakil, M.; Rashedul, H.; Abedin, M. Performance and emission characteristics of a CI engine fueled with Cocos nucifera and Jatropha curcas B20 blends accompanying antioxidants. Ind. Crops Prod. 2014, 57, 132–140. [Google Scholar] [CrossRef]
- Valente, O.S.; Pasa, V.M.D.; Belchior, C.R.P.; Sodré, J.R. Exhaust emissions from a diesel power generator fuelled by waste cooking oil biodiesel. Sci. Total Environ. 2012, 431, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Varatharajan, K.; Cheralathan, M.; Velraj, R. Mitigation of NOx emissions from a jatropha biodiesel fuelled DI diesel engine using antioxidant additives. Fuel 2011, 90, 2721–2725. [Google Scholar] [CrossRef]
- Wang, W.-G.; Lyons, D.W.; Clark, N.N.; Gautam, M.; Norton, P. Emissions from nine heavy trucks fueled by diesel and biodiesel blend without engine modification. Environ. Sci. Technol. 2000, 34, 933–939. [Google Scholar] [CrossRef]
- Arbab, M.I.; Masjuki, H.H.; Varman, M.; Kalam, M.A.; Imtenan, S.; Sajjad, H. Fuel properties, engine performance and emission characteristic of common biodiesels as a renewable and sustainable source of fuel. Renew. Sustain. Energy Rev. 2013, 22, 133–147. [Google Scholar] [CrossRef]
- Ozsezen, A.N.; Canakci, M. Determination of performance and combustion characteristics of a diesel engine fueled with canola and waste palm oil methyl esters. Energy Convers. Manag. 2011, 52, 108–116. [Google Scholar] [CrossRef]
- Gumus, M.; Kasifoglu, S. Performance and emission evaluation of a compression ignition engine using a biodiesel (apricot seed kernel oil methyl ester) and its blends with diesel fuel. Biomass Bioenergy 2010, 34, 134–139. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Robbins, C. Review of the effects of biodiesel on NOx emissions. Fuel Process. Technol. 2012, 96, 237–249. [Google Scholar] [CrossRef]
- Ashok, B.; Nanthagopal, K.; Jeevanantham, A.; Bhowmick, P.; Malhotra, D.; Agarwal, P. An assessment of Calophyllum inophyllum biodiesel fuelled diesel engine characteristics using novel antioxidant additives. Energy Convers. Manag. 2017, 148, 935–943. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; Rodriguez-Fernandez, J. Effect of biodiesel fuels on diesel engine emissions. Prog. Energy Combust. Sci. 2008, 34, 198–223. [Google Scholar] [CrossRef]
- Kinoshita, E.; Myo, T.; Hamasaki, K.; Nishi, S. Combustion Characteristics of Diesel Engine with Coconut Oil Ethyl Ester; SAE Technical Paper; SAE: Warrendale, PA, USA, 2007. [Google Scholar]
- Heywood, J.B. Internal Combustion Engine Fundamentals; Mcgraw-Hill: New York, NY, USA, 1988; Volume 930. [Google Scholar]
- Wang, L.-B.; Yu, H.-Y.; He, X.-H.; Liu, R.-Y. Influence of fatty acid composition of woody biodiesel plants on the fuel properties. J. Fuel Chem. Technol. 2012, 40, 397–404. [Google Scholar] [CrossRef]




| Parameter | Standard Method | Equipment |
|---|---|---|
| Acid value (mg KOH/g oil) | AOCS-Cd 3d-63 [26] | Titration |
| Iodine value (mg/I2/g oil) | AOCS-Cd 1d-92 [26] | Titration |
| Density (kg/cm3) | ASTM 5002 and ASTM D4502 [27] | AMA 4500M, Anton Paar |
| Viscosity (mm2/s) | DIN53015 and DIN12058 [28] | Lovis 2000M, Anton Paar |
| Calorific value (J/g) | DIN51900 and ASTM D4868 [24] | C5000 IKA Werke, Germany |
| Oxidation stability (h) | AOCS-Cd 12b-92 and EN14112 [24] | 873 Rancimat, Metrohm |
| Cetane number | ASTM D 613 [24] | Shatox Octane meter |
| Flash point (°C) | ASTM D 93 [24] | CLA 5, Petrotest |
| Cold filter plugging point (°C) | ASTM D 6371 [24] | FPP 5G’s, ISLby PAC |
| Cloud point and pour point (°C) | ASTM D 2500 and ASTM D 97 [24] | CPP 5G’s |
| Surface tension (Nm) | Pendant drop method | rame hart model 260 |
| CHNS analyser (wt. %) | - | Series II, CHNS/O 2400, Perkin Elmer |
| Fatty acid composition | AOCS- Ce 1-62 [29] | gas chromatographer |
| Triglycerides and glycerine, mono-,di (wt. %) | ASTM D6584 [24] | GC- FID, shimadzu 2010 |
| Properties | CPO | RSO | |
|---|---|---|---|
| Acid value (mg KOH/g oil) | 15.3 ± 0.25 | 60.58 ± 0.16 | |
| Iodine value (mg/I2/g oil) | 54.8 ± 1.18 | 129.8 ± 0.82 | |
| Free fatty acid (%) | 5.96 ± 0.01 | 21.63 ± 0.02 | |
| Density (kg/m3) at 20 °C | 909.7 ± 0.11 | 917.8 ± 0.03 | |
| Viscosity (mm2/s) at 40 °C | 42.2 ± 0.24 | 49.2 ± 0.25 | |
| Calorific value (MJ/kg) | 38.41 ± 0.02 | 37.92 ± 0.01 | |
| Sulfur (wt. %) | 0.751 ± 0.00 | 0.266 ± 0.00 | |
| Nitrogen (wt. %) | 0.419 ± 0.00 | 0.358 ± 0.00 | |
| Glycerides (wt. %) | Monoglycrides | 0.81 ± 0.00 | 6.42 ± 0.16 |
| Diglycerides | 7.64 ± 0.01 | 8.8 ± 0.13 | |
| Triglycerides | 81.8 ± 0.14 | 58.01 ± 0.08 | |
| Fatty acid composition (wt. %) | Myristic acid (C14:0) | 1.13 ± 0.01 | Not defined |
| Palmitic acid (C16:0) | 40.75 ± 0.04 | 9.5 ± 0.02 | |
| Stearic acid (C18:0) | 3.31 ± 0.08 | 7.8 ± 0.40 | |
| Oleic acid (C18:1) | 39.5 ± 0.12 | 25.7 ± 0.23 | |
| Linoleic acid (C18:2) | 9.8 ± 0.13 | 40.6 ± 0.12 | |
| Linolenic acid (C18:3) | n.d. | 15.01 ± 0.123 | |
| Engine Type | 4 Cylinder in Line Diesel Engine, IDI, 4D68 |
|---|---|
| Displacement Volume | 1998 cm3 |
| Bore × Stroke | 82.7 × 93 mm |
| Compression ratio | 22.4:1 |
| Fuel injection pressure | 130 bar |
| Combustion chamber type | Swirl chamber |
| Engine cooling | Water cooling pressurized circulation |
| Dynamometer Specifications | |
| Dynamometer type | SE 150 model |
| Maximum torque, power and speed | 500 Nm, 150 kW and 8000 rpm (standard) |
| Torque and speed calibration accuracy | ±0.25 Nm and ±1 |
| Minimum flow and supply pressure | 107 L/min and 1 kg/cm3 |
| Maximum outlet water temperature | 60 °C |
| Weigh | 560 kg |
| Maximum voltage and current | 250 V AC and 5 A |
| Pulse pick up | Inductive |
| Load cell | Strain gauge-full bridge |
| Energising coil voltage | 90 V |
| Input resistance | 375 ohms |
| Sensitivity and excitation | 2.7 MV/V and 10 V DC |
| Overall size (length × width× height) | 632 × 470 × 689 mm |
| Equipment | Method | Measurement | Upper Limit | Accuracy |
|---|---|---|---|---|
| BOSCH exhaust gas analyzer | Nondispersive infrared | CO | 10.00 vol % | ±0.001 vol % |
| Flame ionization detector | HC | 9999 ppm | ±1 ppm | |
| Nondispersive infrared | CO2 | 18.00 vol % | ±0.01 vol % | |
| Electro-chemical transmitter | NO | 5000 ppm | ±1 ppm |
| Parameters | Measurement Limit | Accuracy | Uncertainty |
|---|---|---|---|
| Torque | 500 Nm | ±0.25 Nm | ±0.86 |
| BSFC | - | ±3.19 g/kWh | ±0.97 |
| Speed | 8000 rpm | ±1 rpm | ±1.40 |
| CO | 10 vol % | ±0.001 vol % | ±1.38 |
| HC | 9999 ppm | ±1 ppm | ±2.49 |
| CO2 | 18 vol % | ±0.01 vol % | ±1.00 |
| NO | 5000 ppm | ±1 ppm | ±1.72 |
| Smoke | 100% | ±0.1% | ±2.16 |
| Pressure | 250 bar | ±0.5 bar | ±0.82 |
| Crank angle encoder | 20000 rpm | ±0.2 rpm | ±0.20 |
| Overall experimental uncertainty | ±5.29% | ||
| Property | RB | PB | RB20 | RB50 | PB20 | PB50 | RPB10 | RPB25 | Diesel | |
|---|---|---|---|---|---|---|---|---|---|---|
| Acid value (mg KOH/g oil) | 0.36 ± 0.21 | 0.30 ± 0.12 | 0.053 ± 0.11 | 0.12 ± 0.16 | 0.041 ± 0.01 | 0.10 ± 0.08 | 0.044 ± 0.13 | 0.10 ± 0.03 | 0.003 ± 0.01 | |
| Density (g/m3) at 20 °C | 0.878 ± 0.20 | 0.862 ± 0.15 | 0.849 ± 0.13 | 0.856 ± 0.11 | 0.831 ± 0.04 | 0.845 ± 0.16 | 0.834 ± 0.22 | 0.847 ± 0.06 | 0.825 ± 0.03 | |
| Viscosity (mm2/s) at 40 °C | 4.2 ± 0.01 | 3.8 ± 0.02 | 3.32 ± 0.01 | 3.65 ± 0.03 | 3.31 ± 0.01 | 3.42 ± 0.11 | 3.31 ± 0.01 | 3.48 ± 0.08 | 3.064 ± 0.02 | |
| Calorific value (MJ/kg) | 39.2 ± 0.03 | 41.8 ± 0.02 | 43.20 ± 0.11 | 41.64 ± 0.02 | 43.88 ± 0.02 | 43.01 ± 0.10 | 43.56 ± 0.02 | 42.87 ± 0.01 | 44.28 ± 0.01 | |
| Cetane number | 51.9 ± 0.05 | 57.5 ± 0.01 | 50.1 ± 0.03 | 50.36 ± 0.44 | 52.3 ± 0.03 | 54.7 ± 08 | 50.8 ± 0.04 | 51 ± 0.05 | 47 ± 0.02 | |
| Oxidation stability (h) | 8.2 ± 0.15 | 25.3 ± 0.10 | 53.52 ± 0.10 | 37.56 ± 0.12 | 85.6 ± 0.05 | 65.98 ± 0.05 | 76.50 ± 0.11 | 59.16 ± 0.01 | 103.6 ± 0.03 | |
| Flash point (°C) | 152 ± 0.36 | 156 ± 0.25 | 86.32 ± 0.25 | 108.74 ± 0.32 | 90.17 ± 0.07 | 114.63 ± 0.30 | 88.93 ± 0.23 | 112.29 ± 0.15 | 72.4 ± 0.11 | |
| Cloud point (°C) | 3.4 ± 0.08 | 11.25 ± 0.13 | −11.8 ± 0.02 | −7.26 ± 0.06 | −10.2 ± 0.02 | −3.69 ± 0.11 | −11.28 ± 0.11 | −7.19 ± 0.01 | −17 ± 0.03 | |
| Pour point (°C) | −2 ± 0.02 | 10.2 ± 0.16 | −22.62 ± 0.22 | −17.74 ± 0.01 | −19.5 ± 0.01 | −9.87 ± 0.13 | −22.48 ± 0.04 | −16.53 ± 0.12 | −32 ± 0.05 | |
| Cold filter plugging point (°C) | 0 ± 0.00 | 10.5 ± 0.12 | −6.93 ± 0.10 | −3.47 ± 0.01 | −3.38 ± 0.05 | −1.2 ± 0.30 | −6.27 ± 0.01 | −3.23 ± 0.07 | −8.7 ± 0.01 | |
| Perkin Elmer, CHNS, 2400 | Carbon (wt. %) | 75.38 ± 0.84 | 77.01 ± 0.20 | 83.36 ± 0.05 | 78.36 ± 0.63 | 84.25 ± 0.03 | 80.82 ± 0.14 | 84.15 ± 0.26 | 80.41 ± 0.11 | 86.62 ± 0.11 |
| Hydrogen (wt. %) | 11.78 ± 0.91 | 12.25 ± 0.28 | 12.61 ± 0.34 | 12.34 ± 0.75 | 13.04 ± 0.21 | 12.84 ± 0.35 | 12.98 ± 0.30 | 12.64 ± 0.36 | 13.21 ± 0.17 | |
| Nitrogen (wt. %) | 0.07 ± 0.01 | 0.11 ± 0.02 | 0.018 ± 0.11 | 0.04 ± 0.01 | 0.024 ± 0.01 | 0.054 ± 0.02 | 0.02 ± 0.00 | 0.043 ± 0.01 | 0.01 ± 0.0 | |
| Sulfur (wt. %) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.13 ± 0.00 | 0.07 ± 0.02 | 0.12 ± 0.01 | 0.07 ± 0.01 | 0.11 ± 0.01 | 0.08 ± 0.01 | 0.16 ± 0.00 | |
| Oxygen (wt. %) | 12.62 ± 0.04 | 12.75 ± 0.02 | 3.15 ± 0.01 | 6.43 ± 0.03 | 3.56 ± 0.01 | 7.24 ± 0.02 | 3.28 ± 0.04 | 6.85 ± 0.02 | 0.0 ± 0.01 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, I.K.; Abdul Aziz, A.R.; Heikal, M.R.; Yusup, S.; Firmansyah; Ahmad, A.S.; Zainal Abidin, E.Z. Performance and Emission Analysis of Rubber Seed, Palm, and Their Combined Blend in a Multi-Cylinder Diesel Engine. Energies 2018, 11, 1522. https://doi.org/10.3390/en11061522
Adam IK, Abdul Aziz AR, Heikal MR, Yusup S, Firmansyah, Ahmad AS, Zainal Abidin EZ. Performance and Emission Analysis of Rubber Seed, Palm, and Their Combined Blend in a Multi-Cylinder Diesel Engine. Energies. 2018; 11(6):1522. https://doi.org/10.3390/en11061522
Chicago/Turabian StyleAdam, Ibrahim Khalil, Abdul Rashid Abdul Aziz, Morgan R. Heikal, Suzana Yusup, Firmansyah, Ahmad Shahrul Ahmad, and Ezrann Zharif Zainal Abidin. 2018. "Performance and Emission Analysis of Rubber Seed, Palm, and Their Combined Blend in a Multi-Cylinder Diesel Engine" Energies 11, no. 6: 1522. https://doi.org/10.3390/en11061522
APA StyleAdam, I. K., Abdul Aziz, A. R., Heikal, M. R., Yusup, S., Firmansyah, Ahmad, A. S., & Zainal Abidin, E. Z. (2018). Performance and Emission Analysis of Rubber Seed, Palm, and Their Combined Blend in a Multi-Cylinder Diesel Engine. Energies, 11(6), 1522. https://doi.org/10.3390/en11061522





