The Effect of Temperature on the Methanogenic Activity in Relation to Micronutrient Availability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethanol Wastewater
2.2. Seed Sludge Preparation
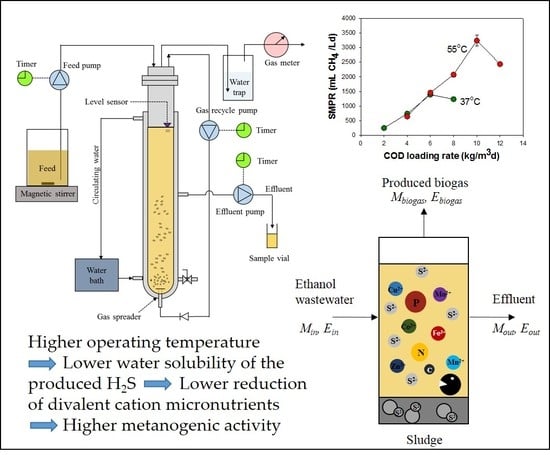
2.3. ASBR Operation
2.4. Measurements and Analytical Methods
3. Results and Discussion
3.1. Characteristics of the As-Used Ethanol Wastewater
3.2. Process Performance Results
3.2.1. Organic Removal
3.2.2. Biogas and CH4 Production Rates
3.2.3. SMPRs and CH4 Yields
3.3. VFA Concentration and Composition
3.4. Alkalinity and pH
3.5. Microbial Concentration and Washout
3.6. Macronutrient Transport
3.7. Micronutrient Transport
3.8. The Mass Balance
3.9. The Energy Balance
3.10. New Explanation of the Methanogenic Activity of Mesophiles and Thermophiles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Nghiem, L.D.; Hai, F.I.; Deng, L.J.; Wang, J.; Wu, Y. Optimization of process parameters for production of volatile fatty acid, biohydrogen and methane from anaerobic digestion. Bioresour. Technol. 2016, 219, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Christy, P.M.; Gopinath, L.; Divya, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew. Sustain. Energy Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Karve, A.D.; Karve, P.; Kulkarni, G. A new compact biogas system based on sugary/starchy feedstock. Energy Sustain. Dev. 2005, 9, 63–65. [Google Scholar] [CrossRef]
- Searmsirimongkol, P.; Rangsunvigit, P.; Leethochawalit, M.; Chavadej, S. Hydrogen production from alcohol distillery wastewater containing high potassium and sulfate using an anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2011, 36, 12810–12821. [Google Scholar] [CrossRef]
- Grimsby, L.K.; Fjørtoft, K.; Aune, J.B. Nitrogen mineralization and energy from anaerobic digestion of jatropha press cake. Energy Sustain. Dev. 2013, 17, 35–39. [Google Scholar] [CrossRef]
- Reungsang, A.; Pattra, S.; Sittijunda, S. Optimization of key factors affecting methane production from acidic effluent coming from the sugarcane juice hydrogen fermentation process. Energies 2012, 5, 4746–4757. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Schmidt, T.; Lv, Z.; Liebetrau, J.; Richnow, H.H.; Kleinsteuber, S.; Nikolausz, M. Reduction of the hydraulic retention time at constant high organic loading rate to reach the microbial limits of anaerobic digestion in various reactor systems. Bioresour. Technol. 2016, 217, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. TRENDS Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Gary, R.K. The concentration dependence of the δs term in the gibbs free energy function: Application to reversible reactions in biochemistry. J. Chem. Educ. 2004, 81, 1599. [Google Scholar] [CrossRef]
- Al-mashhadani, M.K.H.; Wilkinson, S.J.; Zimmerman, W.B. Carbon dioxide rich microbubble acceleration of biogas production in anaerobic digestion. Chem. Eng. Sci. 2016, 156, 24–35. [Google Scholar] [CrossRef]
- Siddique, N.I.; Munaim, M.S.A.; Wahid, Z.A. Role of biogas recirculation in enhancing petrochemical wastewater treatment efficiency of continuous stirred tank reactor. J. Clean. Prod. 2015, 91, 229–234. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Mohanakrishna, G.; Sarma, P.N. Integration of acidogenic and methanogenic processes for simultaneous production of biohydrogen and methane from wastewater treatment. Int. J. Hydrogen Energy 2008, 33, 2156–2166. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; González, J.M.D.; Converti, A.; de Souza Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Intanoo, P.; Chaimongkol, P.; Chavadej, S. Hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactors (UASB) with an emphasis on maximum hydrogen production. Int. J. Hydrogen Energy 2016, 41, 6107–6114. [Google Scholar] [CrossRef]
- Conrad, R. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol. Ecol. 1999, 28, 193–202. [Google Scholar] [CrossRef]
- Prapinagsorn, W.; Sittijunda, S.; Reungsang, A. Co-digestion of napier grass and its silage with cow dung for methane production. Energies 2017, 10, 1654. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Dąbrowska, L. Speciation of heavy metals in sewage sludge after mesophilic and thermophilic anaerobic digestion. Chem. Pap. 2012, 66, 598–606. [Google Scholar] [CrossRef]
- Gao, Y.; Kong, X.; Xing, T.; Sun, Y.; Zhang, Y.; Luo, X.; Sun, Y. Digestion performance and microbial metabolic mechanism in thermophilic and mesophilic anaerobic digesters exposed to elevated loadings of organic fraction of municipal solid waste. Energies 2018, 11, 952. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional mesophilic vs. Thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Gebreeyessus, G.D.; Jenicek, P. Thermophilic versus mesophilic anaerobic digestion of sewage sludge: A comparative review. Bioengineering 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ahn, Y.-H.; Speece, R.E. Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. Thermophilic. Water Res. 2002, 36, 4369–4385. [Google Scholar] [CrossRef]
- Lu, J.; Gavala, H.N.; Skiadas, I.V.; Mladenovska, Z.; Ahring, B.K. Improving anaerobic sewage sludge digestion by implementation of a hyper-thermophilic prehydrolysis step. J. Environ. Manag. 2008, 88, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Gevantman, L.H. The Solubility of Selected Gases in Water. In CRC Handbook of Chemistry and Physics, 72nd ed.; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 1992; pp. 82–83. [Google Scholar]
- Sreethawong, T.; Chatsiriwatana, S.; Rangsunvigit, P.; Chavadej, S. Hydrogen production from cassava wastewater using an anaerobic sequencing batch reactor: Effects of operational parameters, COD: N ratio, and organic acid composition. Int. J. Hydrogen Energy 2010, 35, 4092–4102. [Google Scholar] [CrossRef]
- Eaton, A.; Clesceri, L.; Rice, E.; Greenberg, A. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association (APHA); American Water Works Association (AWWA); Water Enviroment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Pehlivanoglu, E.; Sedlak, D.L. Bioavailability of wastewater-derived organic nitrogen to the alga Selenastrum capricornutum. Water Res. 2004, 38, 3189–3196. [Google Scholar] [CrossRef] [PubMed]
- Intanoo, P.; Rangsunvigit, P.; Namprohm, W.; Thamprajamchit, B.; Chavadej, J.; Chavadej, S. Hydrogen production from alcohol wastewater by an anaerobic sequencing batch reactor under thermophilic operation: Nitrogen and phosphorous uptakes and transformation. Int. J. Hydrogen Energy 2012, 37, 11104–11112. [Google Scholar] [CrossRef]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Xie, L.; Zou, Z.; Wang, W.; Zhou, Q.; Shim, H. Anaerobic treatment of cassava stillage for hydrogen and methane production in continuously stirred tank reactor (CSTR) under high organic loading rate (OLR). Int. J. Hydrogen Energy 2010, 35, 11733–11737. [Google Scholar] [CrossRef]
- Zhu, H.; Stadnyk, A.; Béland, M.; Seto, P. Co-production of hydrogen and methane from potato waste using a two-stage anaerobic digestion process. Bioresour. Technol. 2008, 99, 5078–5084. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Xie, L.; Zhou, Q.; Angelidaki, I. Enhancement of bioenergy production from organic wastes by two-stage anaerobic hydrogen and methane production process. Bioresour. Technol. 2011, 102, 8700–8706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intanoo, P.; Rangsanvigit, P.; Malakul, P.; Chavadej, S. Optimization of separate hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactor (UASB) system under thermophilic operation. Bioresour. Technol. 2014, 173, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Peng, D.; Teng, Z.; Ju, X. Treatment of brewery wastewater using anaerobic sequencing batch reactor (ASBR). Bioresour. Technol. 2008, 99, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Tangkathitipong, P.; Intanoo, P.; Butpan, J.; Chavadej, S. Separate production of hydrogen and methane from biodiesel wastewater with added glycerin by two-stage anaerobic sequencing batch reactors (ASBR). Renew. Energy 2017, 113, 1077–1085. [Google Scholar] [CrossRef]
- Sakar, S.; Yetilmezsoy, K.; Kocak, E. Anaerobic digestion technology in poultry and livestock waste treatment—A literature review. Waste Manag. Res. 2009, 27, 3–18. [Google Scholar] [CrossRef] [PubMed]
- McCarty, P.L.; Rittmann, B.E. Environmental Biotechnology: Principles and Applications; McGraw-Hill: New York, NY, USA, 2001; pp. 19–55. [Google Scholar]
- Li, B.; Wu, G. Effects of sludge retention times on nutrient removal and nitrous oxide emission in biological nutrient removal processes. Int. J. Environ. Res. Public Health 2014, 11, 3553–3569. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Kinyua, M.N. Effect of Solids Retention Time on the Denitrification Potential of Anaerobically Digested Swine Waste. Master’s Thesis, University of South Florida, Tampa, FL, USA, 22 March 2013. [Google Scholar]
- Schattauer, A.; Abdoun, E.; Weiland, P.; Plöchl, M.; Heiermann, M. Abundance of trace elements in demonstration biogas plants. Biosyst. Eng. 2011, 108, 57–65. [Google Scholar] [CrossRef]
- Weiland, P. Anforderungen an pflanzen seitens des biogasanlagenbetreibers, tll-jena, eigenverlag, 12. Thüringer Bioenergietag 2006, 26–32. [Google Scholar]
- Bożym, M.; Florczak, I.; Zdanowska, P.; Wojdalski, J.; Klimkiewicz, M. An analysis of metal concentrations in food wastes for biogas production. Renew. Energy 2015, 77, 467–472. [Google Scholar] [CrossRef]
- Sahm, H. Biologie der methan-bildung. Chemie Ingenieur Technik 1981, 53, 854–863. [Google Scholar] [CrossRef]
- Pobeheim, H.; Munk, B.; Lindorfer, H.; Guebitz, G.M. Impact of nickel and cobalt on biogas production and process stability during semi-continuous anaerobic fermentation of a model substrate for maize silage. Water Res. 2011, 45, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, R.; Ji, M.; Han, L. Hydrogen and methane production by co-digestion of waste activated sludge and food waste in the two-stage fermentation process: Substrate conversion and energy yield. Bioresour. Technol. 2013, 146, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Olsson, G. Water and Energy: Threats and Opportunities; IWA Publishing: London, UK, 2015. [Google Scholar] [CrossRef]
- Shizas, I.; Bagley, D.M. Experimental determination of energy content of unknown organics in municipal wastewater streams. J. Energy Eng. 2004, 130, 45–53. [Google Scholar] [CrossRef]
- DiStefano, T.D.; Palomar, A. Effect of anaerobic reactor process configuration on useful energy production. Water Res. 2010, 44, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Berglund, M.; Börjesson, P. Assessment of energy performance in the life-cycle of biogas production. Biomass Bioenergy 2006, 30, 254–266. [Google Scholar] [CrossRef]





| Chemical Reaction | Equation |
|---|---|
| Hydrolysis: | |
| (1) | |
| Acidogenesis (favorable pH of ~4.5–5.5): | |
| (2) | |
| (3) | |
| (4) | |
| (5) | |
| (6) | |
| (7) | |
| Acetogenesis (favorable pH of ~6): | |
| (8) | |
| (9) | |
| (10) | |
| (11) | |
| (12) | |
| (13) | |
| Methanogenesis (favorable pH of ~7–8): | |
| Hydrogenotrophic methanogenesis: | |
| (14) | |
| Acetotrophic methanogenesis: | |
| (15) | |
| (16) | |
| Parameter | Unit | Value |
|---|---|---|
| pH | - | 3.5 ± 0.2 |
| COD | mg/L | 63,000 ± 900 |
| Total VFA: | mg/L as HAc | 11,100 ± 90 |
| Lactic acid (HLa) | mg/L | 10,500 ± 50 |
| Acetic acid (HAc) | mg/L | 3660 ± 2 |
| Propionic acid (HPr) | mg/L | 300 ± 1 |
| Butylric acid (HBu) | mg/L | 30 ± 1 |
| Valeric acid (HVa) | mg/L | 10 ± 2 |
| Ethanol | mg/L | 540 ± 5 |
| Total solid (TS) | mg/L | 38,200 ± 1800 |
| Total volatile solid (TVS) | mg/L | 34,400 ± 1600 |
| Total carbon (C) | mg/L | 19,000 ± 300 |
| Total nitrogen (N) | mg/L | 640 ± 7 |
| NH4+-N | mg/L | 100 ± 1 |
| NO3−-N | mg/L | 2.0 ± 0.1 |
| NO2−-N | mg/L | 0 |
| Org-N | mg/L | 538 |
| Total P | mg/L | 230 ± 28 |
| Total SO42− | mg/L | 27 ± 1.4 |
| Total S | mg/L | 0.11 |
| COD:N:P:S | - | 100:1:0.4:0.002 |
| Parameters | Micronutrients (ppb) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe2+ | Mn2+ | Zn2+ | Cu2+ | Ni2+ | Mo2+ | Co2+ | ||||||||
| Recommended concentration | 1000–10,000 a 100–400 e | 5–50,000 c 10–50 e | 1000–3000 b 100–1000 e | 60–64,000 c 10–50 e | 5–500 d 50–300 e | 3–50 a | 3–60 a | |||||||
| Feed | 17,500 | 2900 | 1260 | 870 | 150 | 250 | 80 | |||||||
| Effluent at different COD loading rate (kg/m3d) | 37 °C | 55 °C | 37 °C | 55 °C | 37 °C | 55 °C | 37 °C | 55 °C | 37 °C | 55 °C | 37 °C | 55 °C | 37 °C | 55 °C |
| 4 | 1400 | 1000 | 180 | 20 | 12 | 30 | 70 | 30 | 20 | 20 | 0 | 100 | 20 | 20 |
| 6 | 280 | 2250 | 123 | 140 | 15 | 70 | 70 | 150 | 20 | 120 | 40 | 40 | 20 | 80 |
| 8 | 150 | 2300 | 120 | 170 | 10 | 60 | 25 | 120 | 6 | 90 | 0 | 40 | 20 | 40 |
| 10 | - | 350 | - | 25 | - | 30 | - | 110 | - | 30 | - | 40 | - | 20 |
| 12 | - | 270 | - | 10 | - | 30 | - | 70 | - | 40 | - | 0 | - | 20 |
| Sample | Mass Balance (% (w/w) of Feed) | |||
|---|---|---|---|---|
| TS | COD | C | S | |
| Mesophilic ASBR system: | ||||
| Effluent | 35 | 15 | 29 | 0 |
| Biogas | 50 (77%) * | 69 (81%) * | 62 (87%) * | 27 |
| Sludge | 15 (23%) * | 16 (19%) * | 10 (14%) * | 73 |
| Thermophilic ASBR system: | ||||
| Effluent | 13 | 11 | 10 | 0 |
| Biogas | 72 (83%) * | 79 (89%) * | 82 (91%) * | 74 |
| Sludge | 15 (17%) * | 10 (11%) * | 8 (9%) * | 26 |
| Value | Mesophilic ASBR | Thermophilic ASBR |
|---|---|---|
| Energy extraction efficiency (%) | 85 | 92 |
| Energy for bacterial metabolism (%) | 15 | 8 |
| Specific energy production rate (kJ/L d) | 51.6 | 108.7 |
| Specific energy production rate (kJ/gMLVSS d) | 3.6 | 5.2 |
| Energy yield (kJ/g COD applied) | 8.6 | 10.9 |
| Energy yield (kJ/g COD removed) | 9.2 | 12.5 |
| Energy yield (kJ/g TS applied) | 9.9 | 12.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seneesrisakul, K.; Sutabutr, T.; Chavadej, S. The Effect of Temperature on the Methanogenic Activity in Relation to Micronutrient Availability. Energies 2018, 11, 1057. https://doi.org/10.3390/en11051057
Seneesrisakul K, Sutabutr T, Chavadej S. The Effect of Temperature on the Methanogenic Activity in Relation to Micronutrient Availability. Energies. 2018; 11(5):1057. https://doi.org/10.3390/en11051057
Chicago/Turabian StyleSeneesrisakul, Kessara, Twarath Sutabutr, and Sumaeth Chavadej. 2018. "The Effect of Temperature on the Methanogenic Activity in Relation to Micronutrient Availability" Energies 11, no. 5: 1057. https://doi.org/10.3390/en11051057