NOx and SO2 Emissions during Co-Combustion of RDF and Anthracite in the Environment of Precalciner
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Fuels
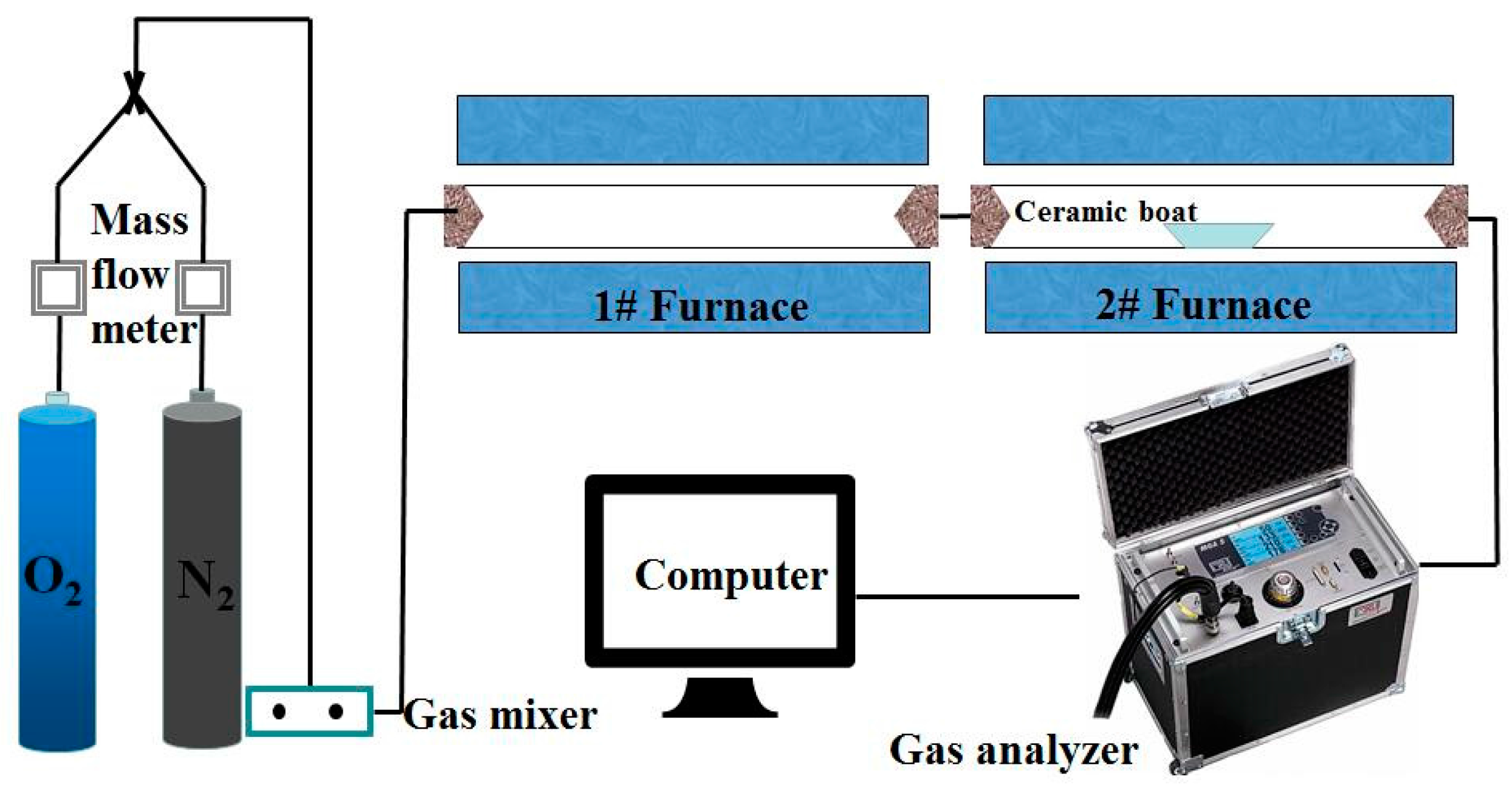
2.2. Apparatus and Methods
3. Results and Discussion
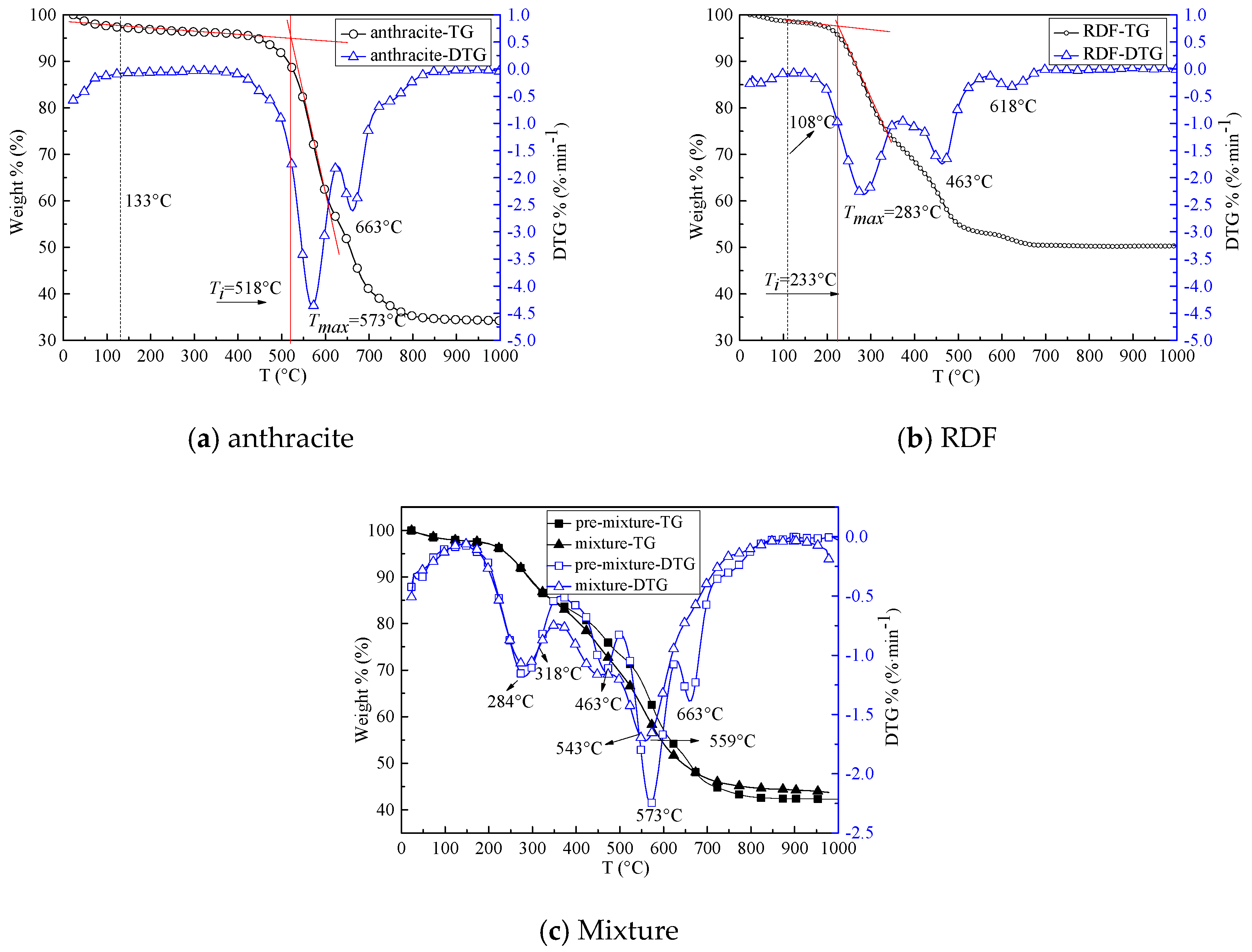
3.1. Combustion Experiments with Thermogravimetric Analyzer (TGA)
- (1)
- Rmax = (dW/dT)max is the maximum weight loss rate, Tmax is the corresponding temperature.
- (2)
- Ti is the ignition temperature.
- (3)
- C = Rmax*106/Ti2 is the flammability index, which shows the reaction capacity in the early stage of combustion.
- (4)
- Rw = 560/Ti + 650/Tmax + 0.27*(dW/dT)max is the ignition stability index.
- (5)
- t0 is the burning-out time.
3.2. Combustion Experiments with a Double Furnaces Reactor
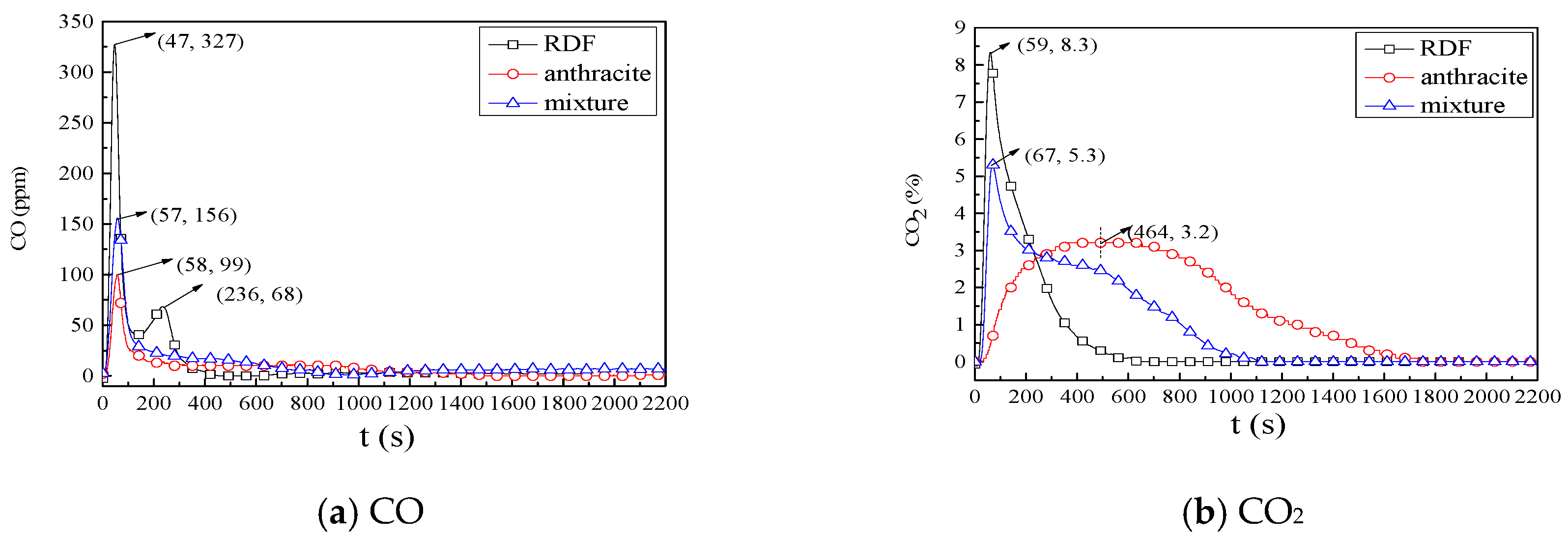
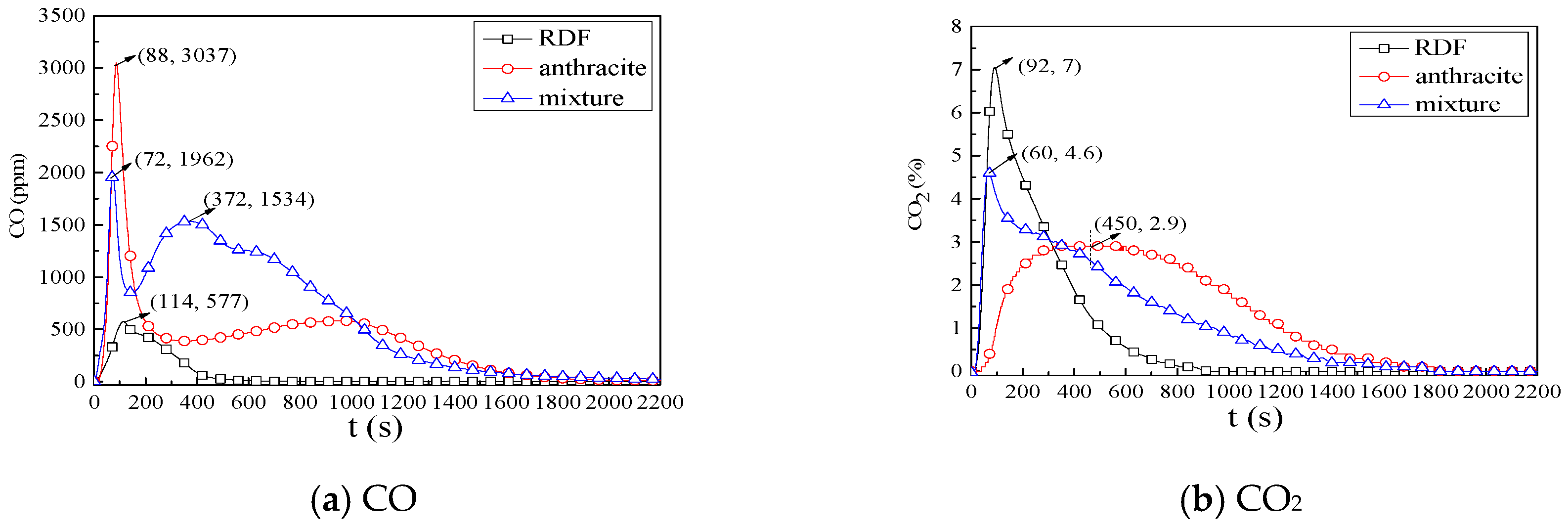
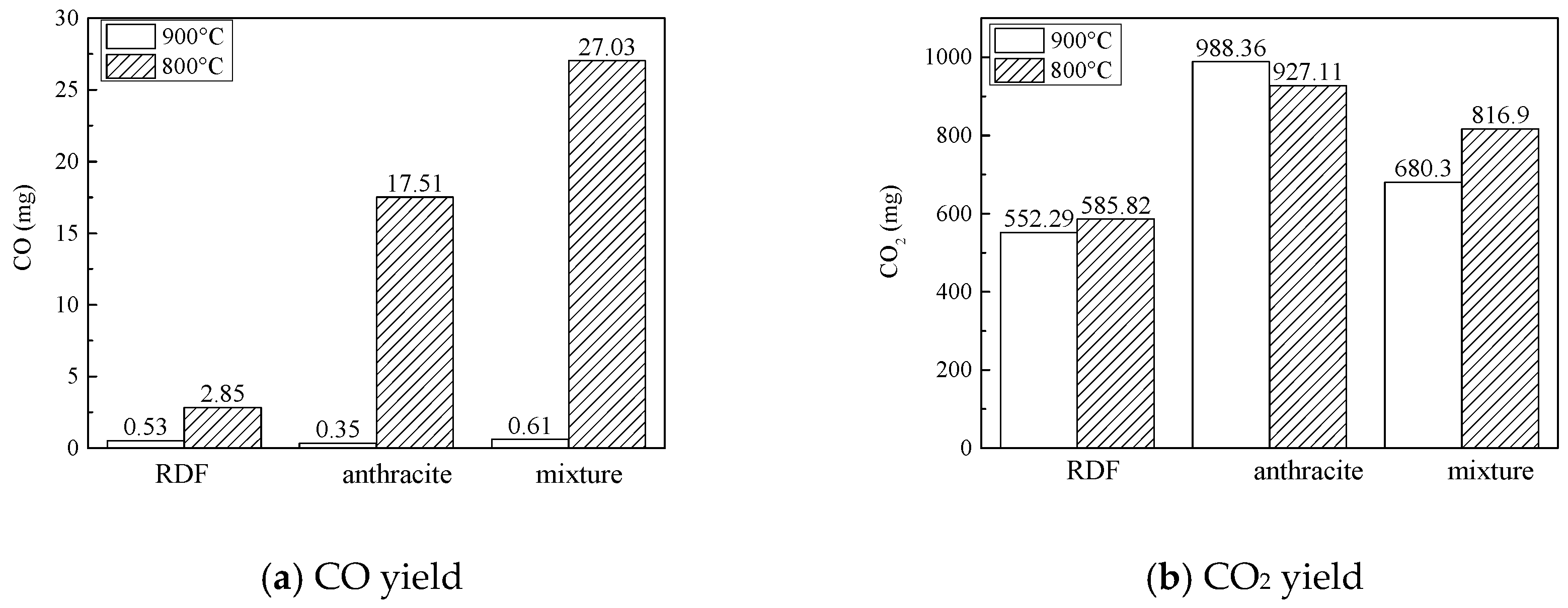
3.2.1. Combustion Process
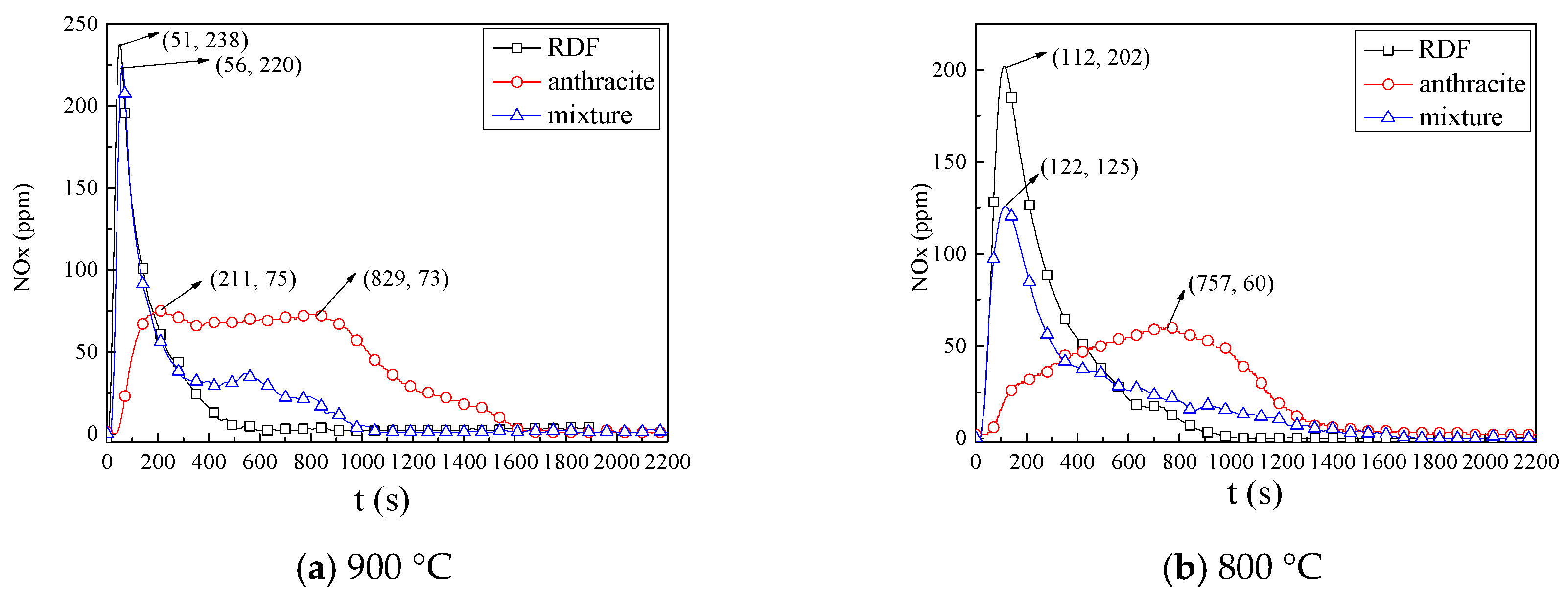
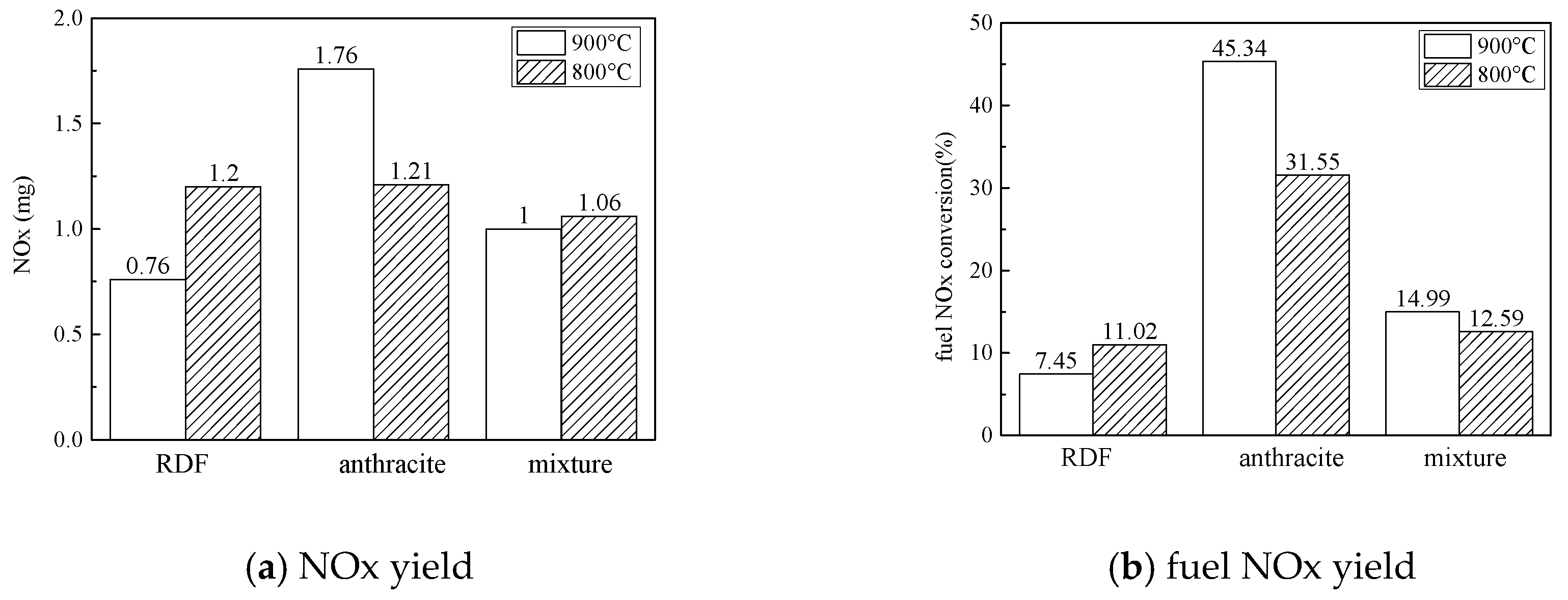
3.2.2. NOx Emission Characteristics
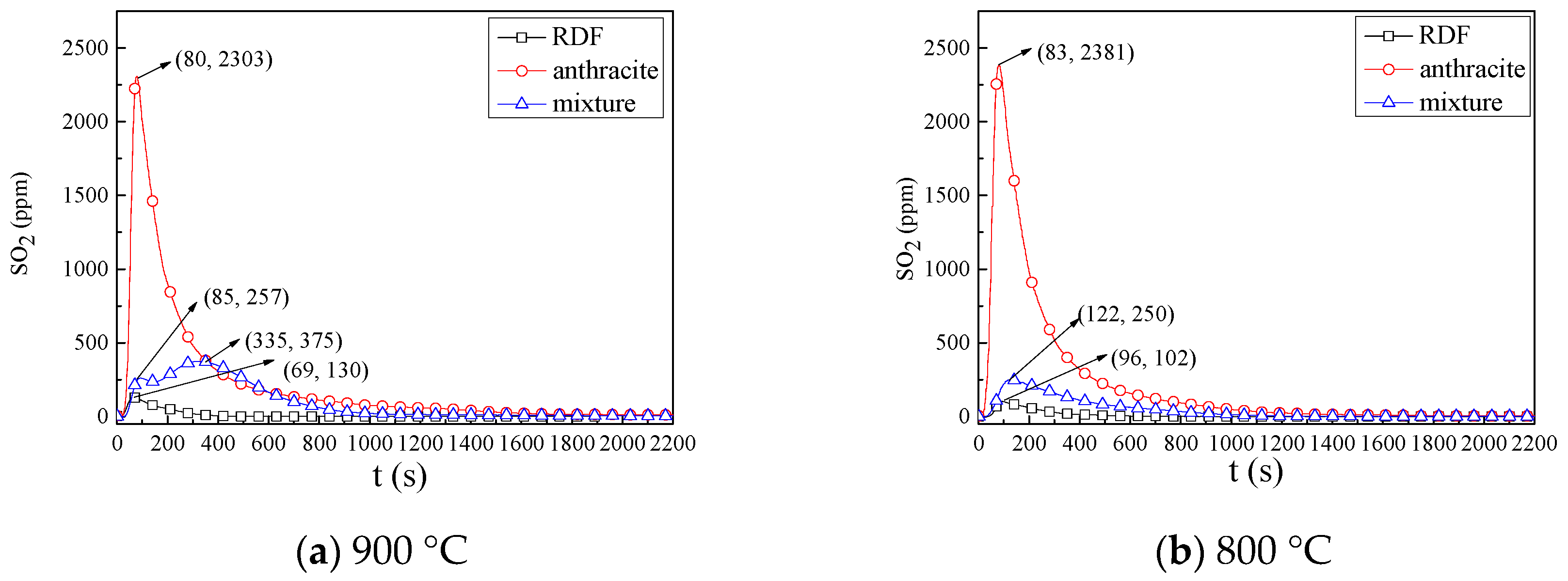
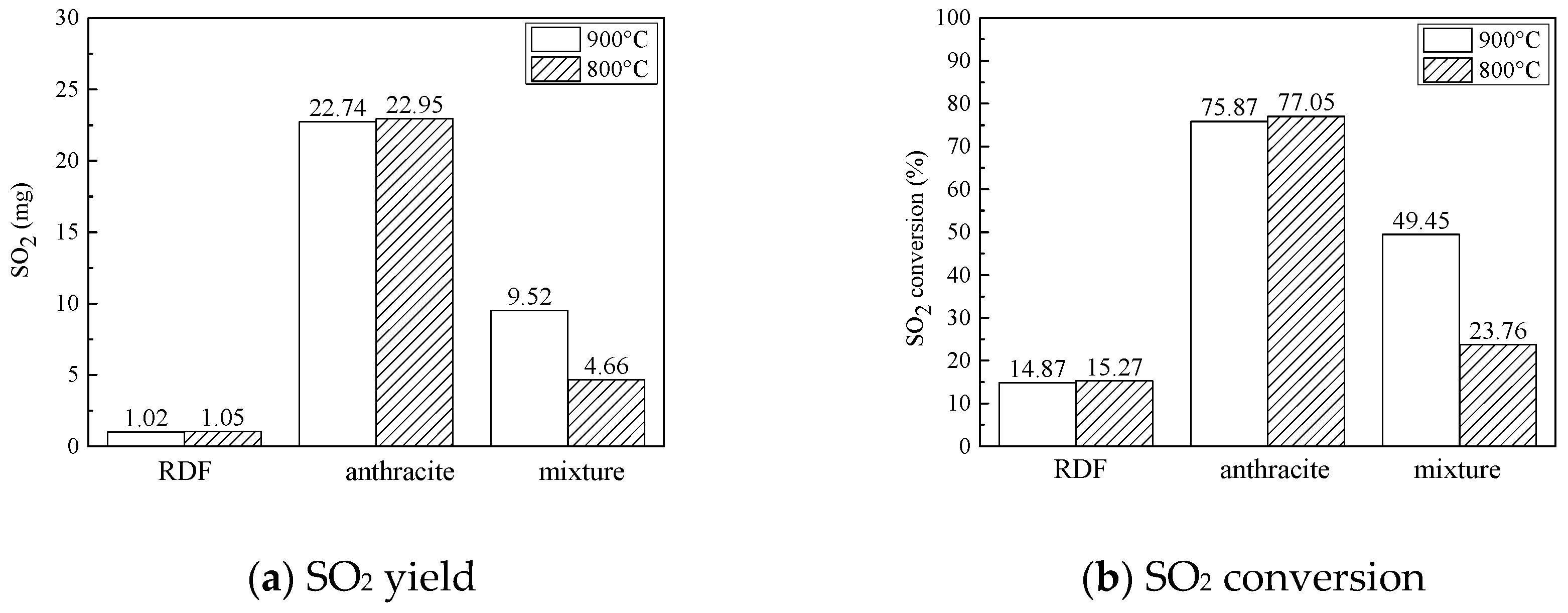
3.2.3. SO2 Emission Characteristics
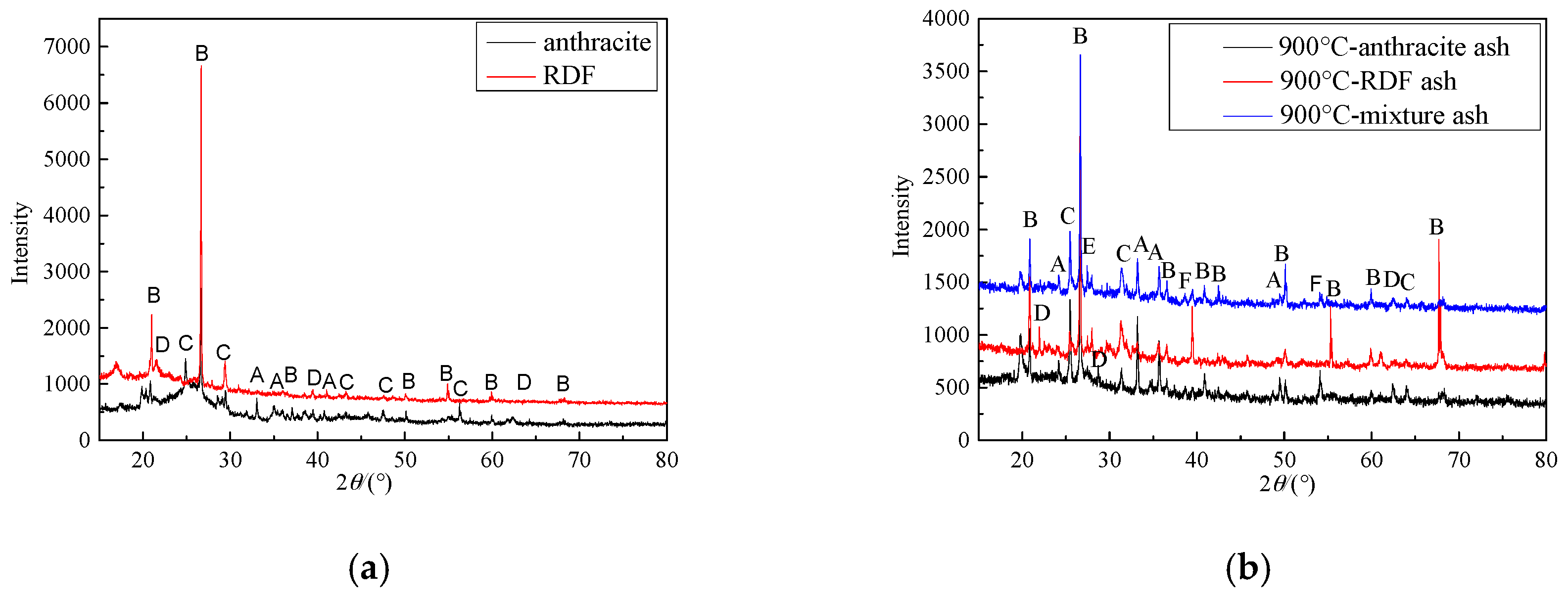
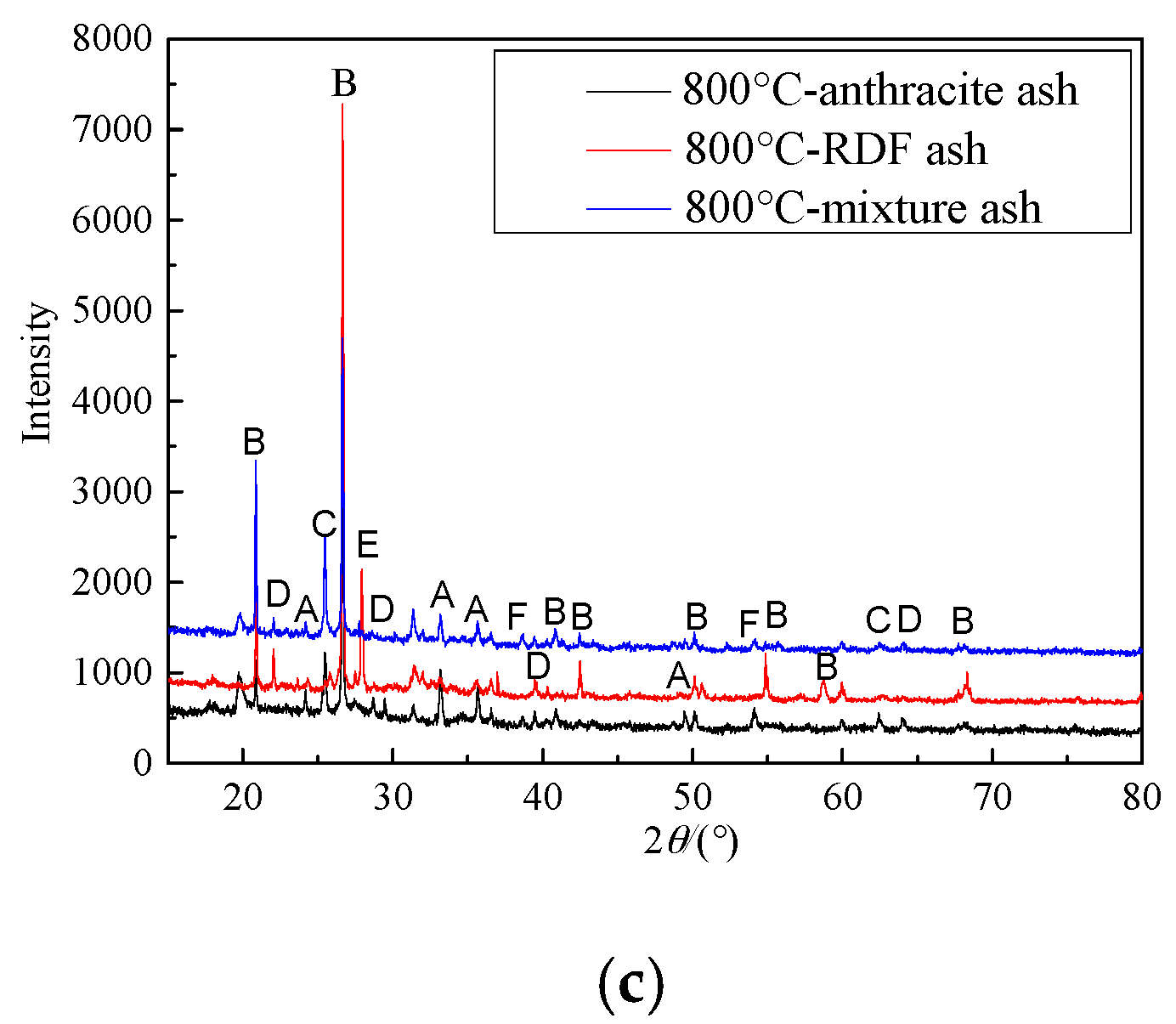
3.3. Ash Characteristics
4. Conclusions
- (1)
- The combustion characteristics: The combustion process of RDF is dominated by devolatilization and volatile matter combustion, whose ignition temperature is low and whose burn-out time is short; the combustion process of anthracite is dominated by char combustion, whose ignition temperature is high and whose burn-out time is long; in the early stage of co-combustion, constituting the linear additive effect of the separate combustion of RDF and anthracite, the later co-combustion stage constitutes the synergistic effect. It is feasible to use the mixture of RDF and anthracite as an alternative fuel in the cement plant.
- (2)
- The temperature effect on the co-combustion process: At 900 °C, the synergistic effect is obvious in the char combustion stage, which could promote the combustion of anthracite and shorten the burn-out time; at 800 °C, the combustion rate is low in the whole combustion process, and the burn-out time of the co-combustion is nearly the same as the time of the separate combustion of anthracite.
- (3)
- NOx emission characteristics: During co-combution, at 900 °C, NOx released rapidly during the devolatilization stage, but in the char combustion stage the NOx formation were inhibited; at 800 °C, a large amount of CO formed, which could reduce the NOx. In general, at 900 °C and at 800 °C, the application of co-combustion could lower the NOx emission yield and lower the NOx conversion.
- (4)
- SO2 emission characteristics: Combined the combustion characteristics and the XRD results, it was indicated that during co-combustion, at 800 °C, the SO2 formation reaction was inhibited by metal oxides, and the SO2 yield and conversion were quite low.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vesterinen, R.; Flyktman, M. Organic emissions from co-combustion of RDF with wood chips and milled peat in a bubbling fluidized bed boiler. Chemosphere 1996, 32, 681–689. [Google Scholar] [CrossRef]
- Zhu, T.Y.; Ye, M.; Jing, P.F.; Xu, W.Q.; Xiao, Y.H. Properties of flue gas from mixed incineration of municipal solid waste and coal in a 1.5 MW circulating fluidized bed boiler. CIESC J. 2010, 61, 2468–2473. [Google Scholar]
- Bai, J.S.; Yu, C.J.; Li, L.M.; LI, X.L.; Wang, Q.H.; Luo, Z.Y. Experimental Investigation on Co-firing of Coal and Refuse-derived Fuel in a Pilot-scale Circulating Fluidized Bed Combustor. Proc. CSEE 2012, 32, 36–41. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, D.W.; Kim, J.S.; Na, J.G.; Lee, S.H. Co-combustion of refuse derived fuel with Korean anthracite in a commercial circulating fluidized bed boiler. Energy 2010, 35, 2814–2818. [Google Scholar] [CrossRef]
- Li, X.G.; Lv, Y.; Ma, B.G.; Jian, S.W.; Tan, H.B. Thermogravimetric investigation on co-combustion characteristics of tobacco residue and high-ash anthracite coal. Bioresour. Technol. 2011, 102, 9783–9787. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.Y.; Zhu, T.L.; Sun, Y.F.; Dong, L. Effects of gas compositions on NOx reduction by selective non-catalytic reduction with ammonia in a simulated cement precalciner atmosphere. Chemosphere 2014, 113, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Mikulčić, H.; Berg, E.V.; Vujanović, M.; Priesching, P.; Tatschl, R.; Duic, N. Numerical analysis of cement calciner fuel efficiency and pollutant emissions. Clean Technol. Environ. 2013, 15, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.F.; Liu, Y.H.; Liu, Y.H.; Che, D.F. N2 formation from coal nitrogen during pyrolysis and combustion. J. Xian Jiaotong Univ. 2008, 42, 350–353. [Google Scholar]
- Gong, Z.Q.; Liu, Z.C.; Zhu, Z.P.; Yu, K.S.; Meng, G.J.; Liu, J.P.; Ouyang, Z.Q.; Sun, Y.K.; Lv, Q.G. Experimental study on semi-coke combustion and coal pyrolysis and combustion coupling. J. China Coal Soc. 2014, 39, 519–525. [Google Scholar]
- Zhou, H.; Huang, Y.; Mo, G.Y.; Liao, Z.Y.; Cen, K.F. Conversion of Fuel-N to N2O and NOx during Coal Combustion in Combustors of Different Scale. Chin. J. Chem. Eng. 2013, 21, 999–1006. [Google Scholar] [CrossRef]
- Collings, M.E.; Mann, M.D.; Young, B.C. Effect of coal rank and circulating fluidized-bed operating parameters on nitrous oxide emissions. Energy Fuels 1993, 7, 554–558. [Google Scholar] [CrossRef]
- Bo, L. Fluidized bed combustion: Mixing and pollutant limitation. Prog. Energy Combust. 1998, 24, 31–61. [Google Scholar] [CrossRef]
- De, D.L.F.; Londono, C.A.; Wang, X.S.; Gibbs, B.M. Influence of operating parameters on NOx and N2O axial profiles in a circulating fluidized bed combustor. Fuel 1996, 75, 971–978. [Google Scholar] [CrossRef]
- Lin, Z.M.; Wang, Z.H.; Liu, J.; Ge, L.C.; Zhang, Y.W.; Zhang, Q.; Zhou, J.H.; Cen, K.F. Experimental study on combustion and NOx emission characteristics of Zhehun blended coal. Therm. Power Gen. 2014, 43, 44–48. [Google Scholar]
- Chui, E.H.; Majeski, A.J.; Douglas, M.A.; Tan, Y.; Thambimuthu, K.V. Numerical investigation of oxy-coal combustion to evaluate burner and combustor design concepts. Energy 2004, 29, 1285–1296. [Google Scholar] [CrossRef]
- Muto, M.; Watanabe, H.; Kurose, R.; Komori, S.; Balusamy, S.; Hochgreb, S. Large-eddy simulation of pulverized coal jet flame—Effect of oxygen concentration on NOx formation. Fuel 2015, 142, 152–163. [Google Scholar] [CrossRef]
- Christopher, R.S.; Alejandro, M. Fundamental investigation of NOx formation during oxy-fuel combustion of pulverized coal. P. Combust. Inst. 2011, 33, 1723–1730. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Zhou, N.; Chen, Y.J.; Zhou, J.H.; Liu, J.Z.; Cen, K.F. Experimental Research on the Combustion and NOx Generation Characteristics of Low Volatile Coal. Proc. CSEE 2010, 30, 55–61. [Google Scholar]
- Liu, Z.G.; Quek, A.; Hoekman, S.K.; Srinivasan, M.P.; Balasubramanian, R. Thermogravimetric investigation of hydrochar-lignite co-combustion. Bioresour. Technol. 2012, 123, 646–652. [Google Scholar] [CrossRef] [PubMed]
| Samples | Proximate Analysis/wt % | Ultimate Analysis/wt % | Qnet,daf/MJ·kg−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | Fcad | Cad | Had | Oad | Nad | Sad | ||
| RDF | 3.60 | 48.75 | 46.9 | 0.75 | 36.78 | 4.36 | 4.57 | 1.16 | 0.78 | 12.42 |
| anthracite | 2.70 | 36.16 | 8.39 | 52.75 | 55.26 | 1.32 | 3.81 | 0.38 | 3.07 | 19.59 |
| Samples | Anthracite | RDF | Mixture | Pre-Mixture |
|---|---|---|---|---|
| Ti (°C) | 518 | 233 | 318 | 398 |
| Rmax (mg·min−1) | 4.36 | 2.31 | 1.72 | 2.24 |
| C (mg·min−1·°C−1) | 16.25 | 42.55 | 17.01 | 14.14 |
| Tmax (°C) | 573 | 283 | 559 | 573 |
| Rw (°C−1) | 3.39 | 5.32 | 3.39 | 3.15 |
| Loss weight (%) | 65.74 | 49.48 | 55.61 | 57.27 |
| t0 (min) | 81.63 | 67.47 | 78.03 | 80.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Xie, J.; Mei, S.; He, F. NOx and SO2 Emissions during Co-Combustion of RDF and Anthracite in the Environment of Precalciner. Energies 2018, 11, 337. https://doi.org/10.3390/en11020337
Chen X, Xie J, Mei S, He F. NOx and SO2 Emissions during Co-Combustion of RDF and Anthracite in the Environment of Precalciner. Energies. 2018; 11(2):337. https://doi.org/10.3390/en11020337
Chicago/Turabian StyleChen, Xiaolin, Junlin Xie, Shuxia Mei, and Feng He. 2018. "NOx and SO2 Emissions during Co-Combustion of RDF and Anthracite in the Environment of Precalciner" Energies 11, no. 2: 337. https://doi.org/10.3390/en11020337




