Influence of Conductive and Semi-Conductive Nanoparticles on the Dielectric Response of Natural Ester-Based Nanofluid Insulation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Sample Characterisation Techniques
2.4. Dielectric Response Measurements
3. Results
3.1. Sample Characterisation Results
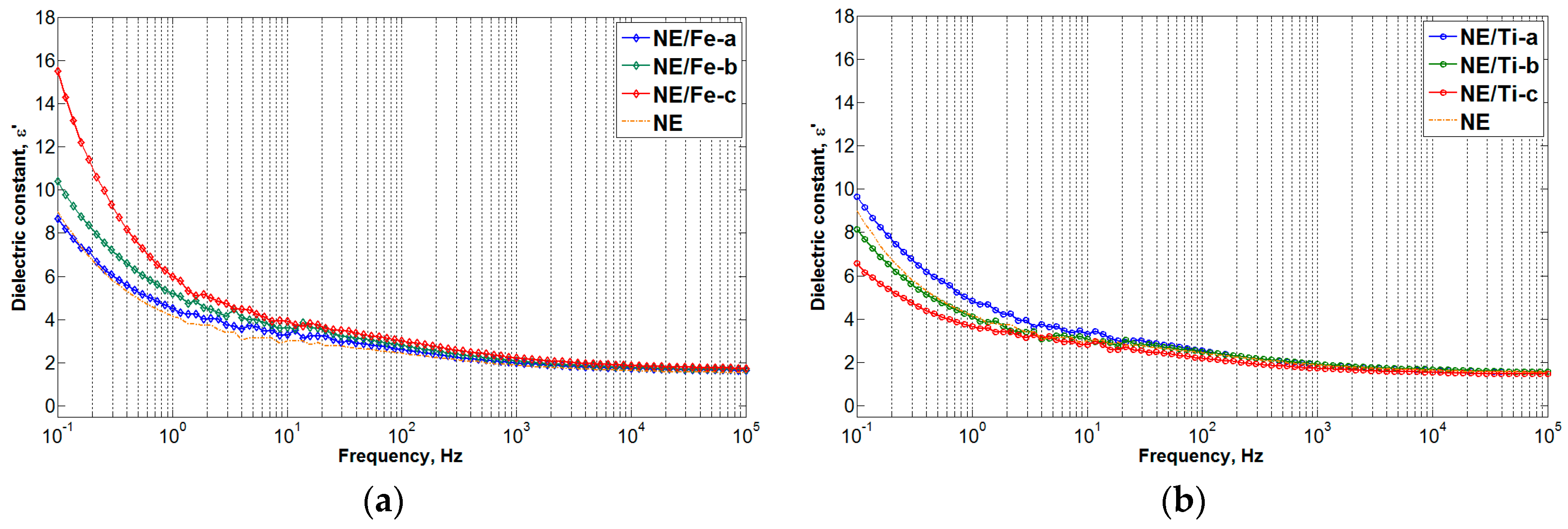
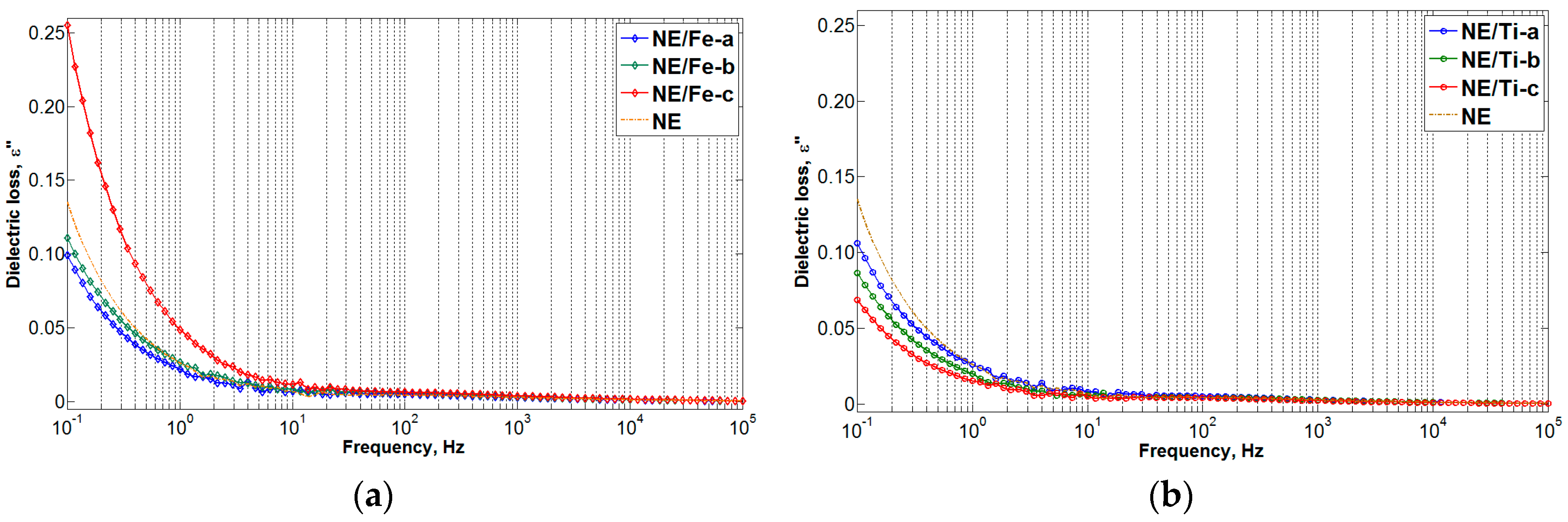
3.2. Frequency Dielectric Response (FDS) Results
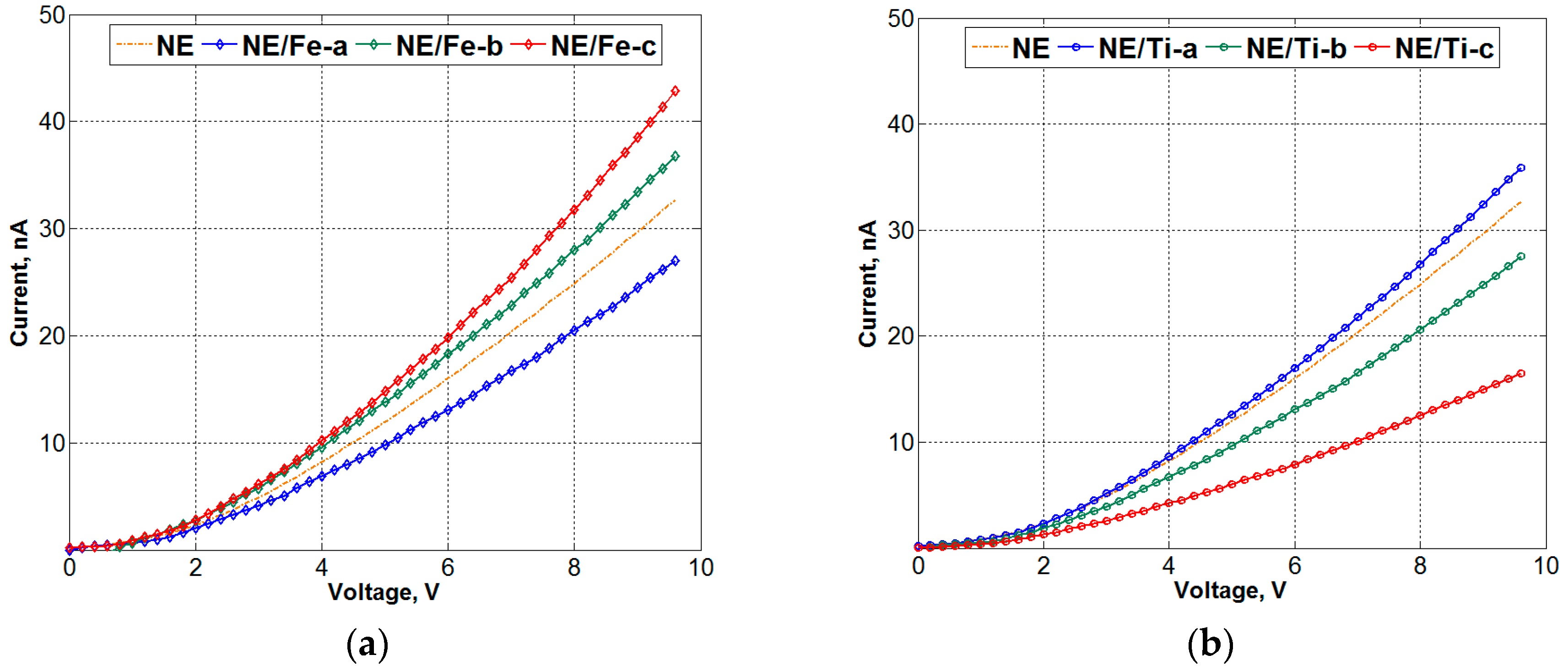
3.3. Current-Voltage (I-V) Results
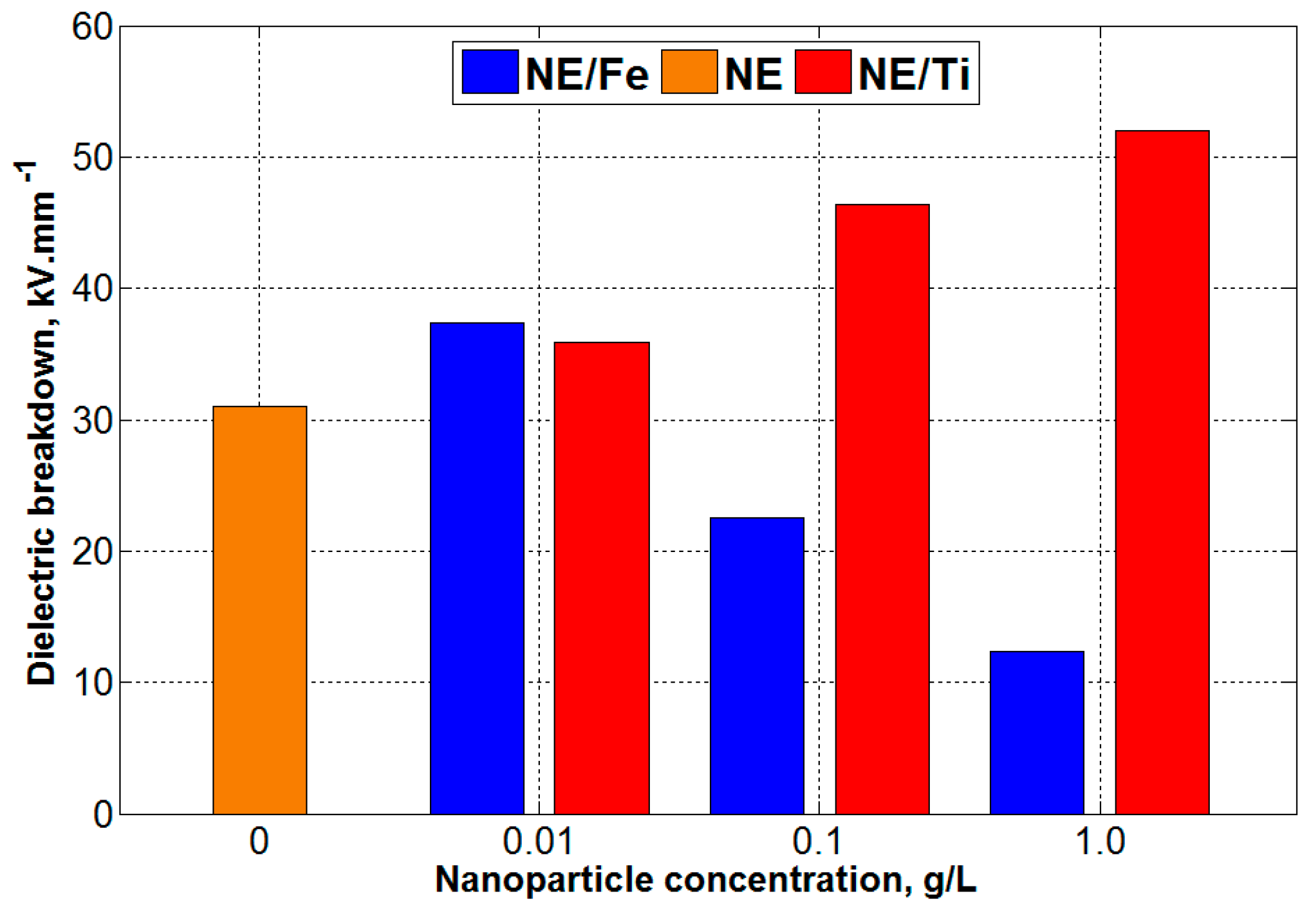
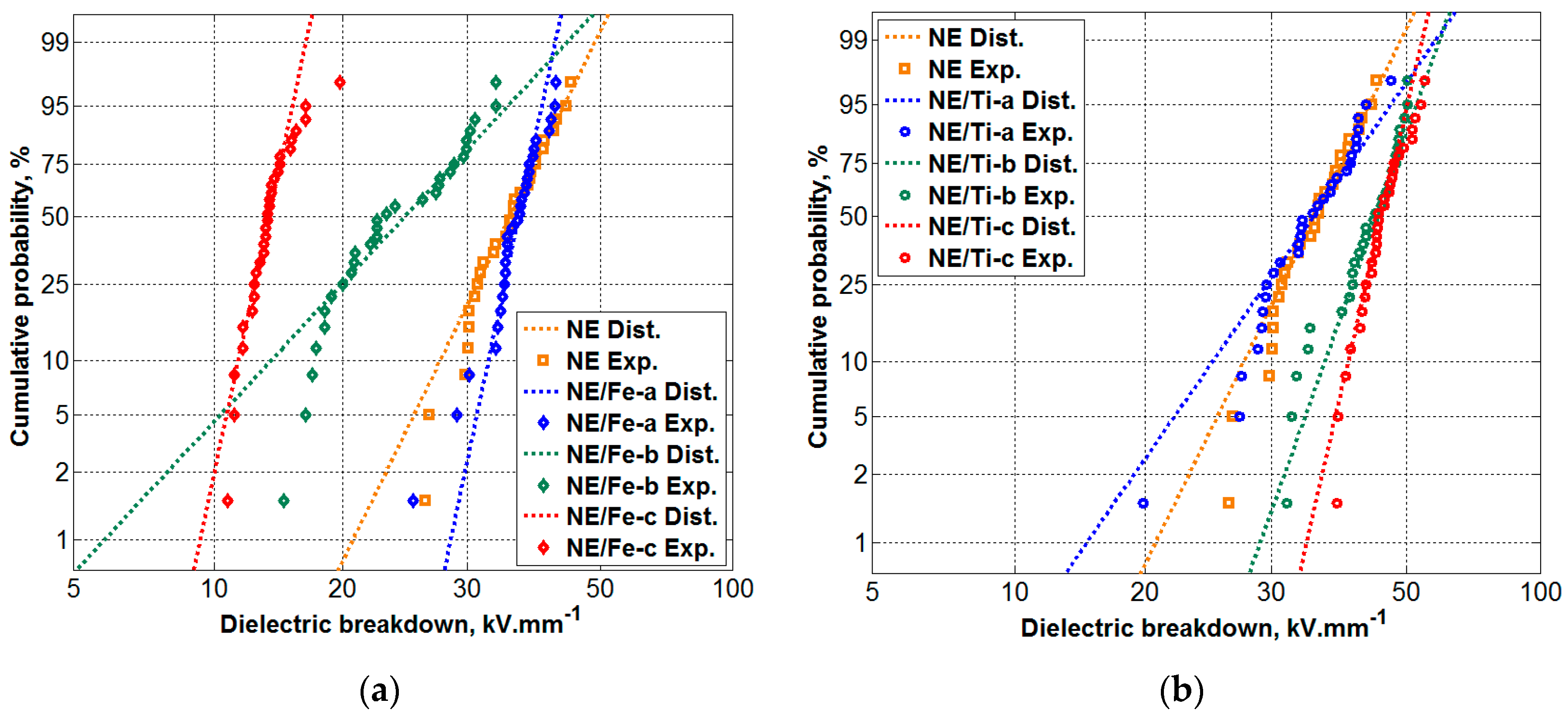
3.4. Dielectric Breakdown Voltage (BDV) Results
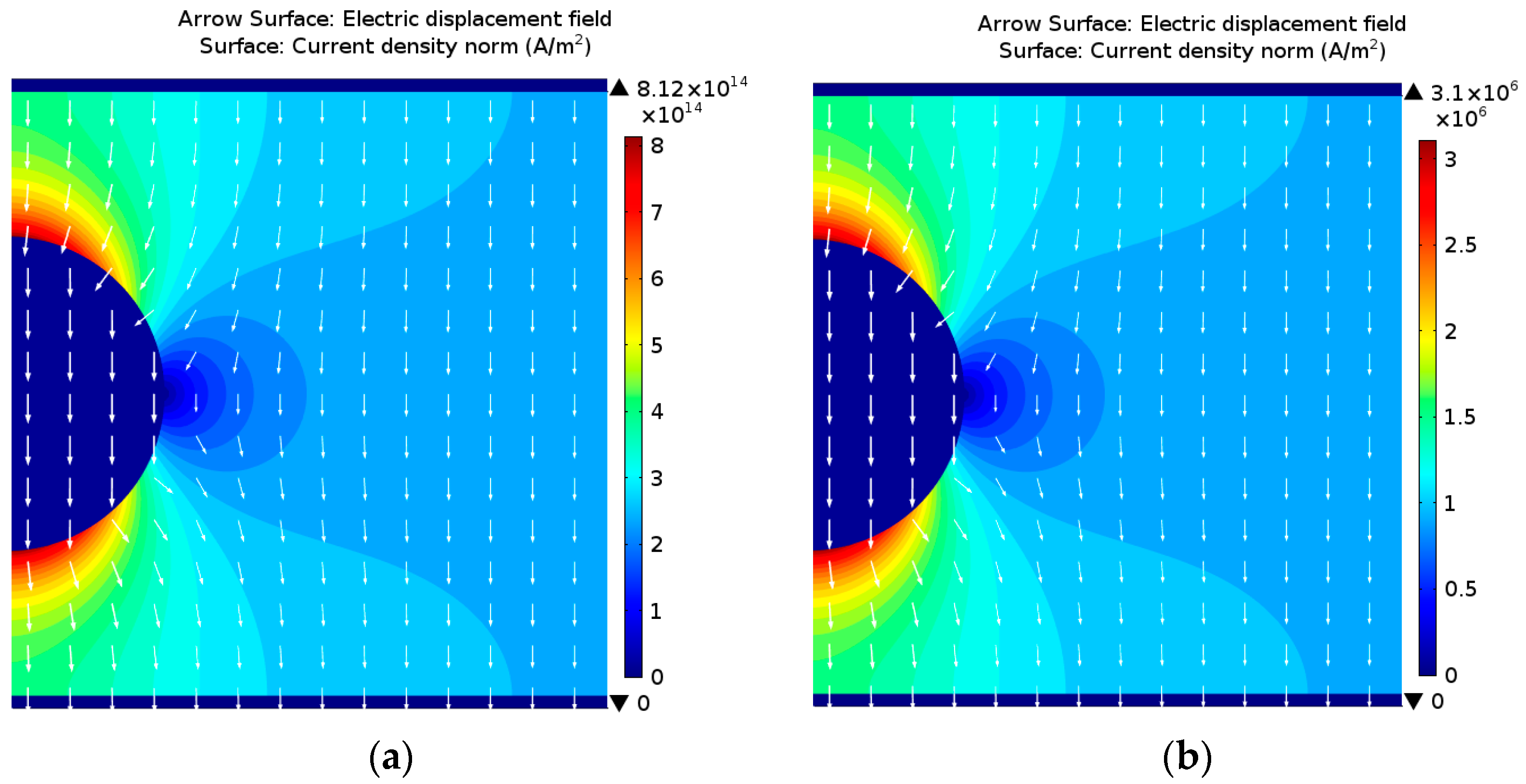
4. Discussion
5. Conclusions
- Natural ester, such as palm oil, which consists of natural oleic acid as a substantial content, produces a stable nanofluid suspension when dispersed with metal oxide nanoparticles. Oleic acid plays an important role as an artificial surfactant, which enhances the suspension stability and avoids agglomeration.
- At 0.01 g/L concentration of nanoparticles within natural ester, the effect of the interfacial zone was dominant and a large space for rigid intermolecular forces was created, requiring more energy to break up. This behaviour was shown through the reduction of dielectric loss and the enhancement of dielectric strength for both types of nanoparticles.
- When the concentration of nanoparticles within natural ester was increased to 0.1 and 1.0 g/L, respectively, the dielectric response of nanofluids became more dependent on the nanoparticle properties (in this case, surface conductivity). Since iron oxide is highly conductive compared to titanium dioxide, the enhancement of the polarization and conduction current was observed with increasing concentration of conductive nanoparticles. This has also led to the reduction of dielectric breakdown voltage.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McShane, C.P. Relative properties of the new combustion-resist vegetable-oil-based dielectric coolants for distribution and power transformers. IEEE Trans. Ind. Appl. 2001, 37, 1132–1139. [Google Scholar] [CrossRef]
- McShane, C.P.; Corkran, J.; Rapp, K.; Luksich, J. Natural Ester Dielectric Fluid Development. In Proceedings of the 2005/2006 IEEE PES Transmission and Distribution Conference and Exhibition, Dallas, TX, USA, 21–24 May 2006; pp. 18–22. [Google Scholar]
- Mehta, D.M.; Kundu, P.; Chowdhury, A.; Lakhiani, V.K.; Jhala, A.S. A review of critical evaluation of natural ester vis-a-vis mineral oil insulating liquid for use in transformers: Part II. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1705–1712. [Google Scholar] [CrossRef]
- Kiasatina, K.; Kamarol, M.; Zulhilmey, M.; Arief, Y.A. Breakdown characteristics of RBDPO and soybean oil mixture for transformer application. In Proceedings of the 2011 International Conference on Electrical, Control and Computer Engineering (INECCE), Pahang, Malaysia, 21–22 June 2011; pp. 219–222. [Google Scholar]
- Beroual, A.; Khaled, U.; Mbolo Noah, P.; Sitorus, H. Comparative Study of Breakdown Voltage of Mineral, Synthetic and Natural Oils and Based Mineral Oil Mixtures under AC and DC Voltages. Energies 2017, 10, 511. [Google Scholar] [CrossRef]
- Abdelmalik, A.A. Chemically modified palm kernel oil ester: A possible sustainable alternative insulating fluid. Sustain. Mater. Technol. 2014, 1–2, 42–51. [Google Scholar] [CrossRef]
- Bandara, K.; Ekanayake, C.; Saha, T.; Ma, H. Performance of natural ester as a transformer oil in moisture-rich environments. Energies 2016, 9, 258. [Google Scholar] [CrossRef]
- Suleiman, A.A.; Muhamad, N.A.; Bashir, N.; Murad, N.S.; Arief, Y.Z.; Phung, B.T. Effect of moisture on breakdown voltage and structure of palm based insulation oils. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 2119–2126. [Google Scholar] [CrossRef]
- Lv, Y.Z.; Zhou, Y.; Li, C.R.; Wang, Q.; Qi, B. Recent progress in nanofluids based on transformer oil: Preparation and electrical insulation properties. IEEE Electr. Insul. Mag. 2014, 30, 23–32. [Google Scholar] [CrossRef]
- Amiri, A.; Kazi, S.N.; Shanbedi, M.; Mohd Zubir, M.N.; Yarmand, H.; Chew, B.T. Transformer oil based multi-walled carbon nanotube-hexylamine coolant with optimized electrical, thermal and rheological enhancements. RSC Adv. 2015, 5, 107222–107236. [Google Scholar] [CrossRef]
- Sima, W.; Shi, J.; Yang, Q.; Huang, S.; Cao, X. Effects of conductivity and permittivity of nanoparticle on transformer oil insulation performance: experiment and theory. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 380–390. [Google Scholar] [CrossRef]
- Nazari, M.; Rasoulifard, M.H.; Hosseini, H. Dielectric breakdown strength of magnetic nanofluid based on insulation oil after impulse test. J. Magn. Magn. Mater. 2016, 399, 1–4. [Google Scholar] [CrossRef]
- Makmud, M.Z.H.; Illias, H.A.; Chee, C.Y. Partial Discharge Behaviour within Palm Oil-based Fe2O3 Nanofluids under AC Voltage. IOP Conf. Ser. Mater. Sci. Eng. 2017, 210, 12034. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Li, J.; Ran, H.; Huang, D. Influence of copper particles on breakdown voltage and frequency-dependent dielectric property of vegetable insulating oil. Energies 2017, 10, 938. [Google Scholar] [CrossRef]
- Kopčanský, P.; Tomčo, L.; Marton, K.; Koneracká, M.; Timko, M.; Potočová, I. The DC dielectric breakdown strength of magnetic fluids based on transformer oil. J. Magn. Magn. Mater. 2005, 289, 415–418. [Google Scholar] [CrossRef]
- Wang, Q.; Rafiq, M.; Lv, Y.; Li, C.; Yi, K. Preparation of three types of transformer oil-based nanofluids and comparative study on the effect of nanoparticle concentrations on insulating property of transformer oil. J. Nanotechnol. 2016, 2016, 5802753. [Google Scholar] [CrossRef]
- Nelson, J.K. Dielectric Polymer Nanocomposites, 1st ed.; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-1591-7. [Google Scholar]
- Atiya, E.G.; Mansour, D.-E.A.; Khattab, R.M.; Azmy, A.M. Dispersion behavior and breakdown strength of transformer oil filled with TiO2 nanoparticles. IEEE Trans. Dielectr. Electr. Insul. 2015, 22. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Olsson, R.T.; Hedenqvist, M.S. The Role of Interfaces in Polyethylene/Metal-Oxide Nanocomposites for Ultrahigh-Voltage Insulating Materials. Adv. Mater. 2017, 30, 1703624. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.E.; Abd-Elhady, A.M.; Izzularab, M.A. Effect of nanoparticles on transformer oil breakdown strength: Experiment and theory. IET Sci. Meas. Technol. 2016, 10, 839–845. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Pourrahimi, A.M.; Hedenqvist, M.S.; Sjöstedt, C.; Bellander, M.; Gedde, U.W. Degradation of carbon-black-filled acrylonitrile butadiene rubber in alternative fuels: Transesterified and hydrotreated vegetable oils. Polym. Degrad. Stab. 2016, 123, 69–79. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Pallon, L.K.H.; Liu, D.; Hoang, T.A.; Gubanski, S.; Hedenqvist, M.S.; Olsson, R.T.; Gedde, U.W. Polyethylene Nanocomposites for the Next Generation of Ultralow-Transmission-Loss HVDC Cables: Insulation Containing Moisture-Resistant MgO Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14824–14835. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, A.; Saidur, R.; Metselaar, H.S.C. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Liu, D.; Pourrahimi, A.M.; Pallon, L.K.H.; Sánchez, C.C.; Olsson, R.T.; Hedenqvist, M.S.; Fogelström, L.; Malmström, E.; Gedde, U.W. Interactions between a phenolic antioxidant, moisture, peroxide and crosslinking by-products with metal oxide nanoparticles in branched polyethylene. Polym. Degrad. Stab. 2016, 125, 21–32. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Liu, D.; Andersson, R.L.; Ström, V.; Gedde, U.W.; Olsson, R.T. Aqueous Synthesis of (210) Oxygen-Terminated Defect-Free Hierarchical ZnO Particles and Their Heat Treatment for Enhanced Reactivity. Langmuir 2016, 32, 11002–11013. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Dong, M.; Ren, M.; Wu, X.; Shen, L.; Wang, H. Effect of nanoparticle polarization on relative permittivity of transformer oil-based nanofluids. J. Appl. Phys. 2013, 113, 204103. [Google Scholar] [CrossRef]
- Negri, F.; Cavallini, A. Analysis of conduction currents in nanofluids. In Proceedings of the 2015 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Ann Arbor, MI, USA, 18–21 October 2015; pp. 27–30. [Google Scholar]
- Hwang, J.G.; Zahn, M.; O’Sullivan, F.M.; Pettersson, L.A.A.; Hjortstam, O.; Liu, R. Effects of nanoparticle charging on streamer development in transformer oil-based nanofluids. J. Appl. Phys. 2010, 107, 014310. [Google Scholar] [CrossRef]
- Jadidian, J.; Zahn, M.; Lavesson, N.; Widlund, O.; Borg, K. Effects of impulse voltage polarity, peak amplitude, and rise time on streamers initiated from a needle electrode in transformer oil. IEEE Trans. Plasma Sci. 2012, 40, 909–918. [Google Scholar] [CrossRef]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orru, S.; Buono, P. Biological and nutritional properties of palm oil and palmitic acid: Effects on health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Fu, L.; Su, M.; Aslam, M.; Wong, K.C.; Dravid, V.P. Interaction of Fatty Acid Monolayers with Cobalt Nanoparticles. Nano Lett. 2004, 4, 383–386. [Google Scholar] [CrossRef]
- Mansour, D.-E.A.; Elsaeed, A.M.; Izzularab, M.A. The role of interfacial zone in dielectric properties of transformer oil-based nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3364–3372. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhong, Y.; Zhou, J.; Li, X.; Zhou, Y. Effect of semiconductive nanoparticles on insulating performances of transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 770–776. [Google Scholar] [CrossRef]
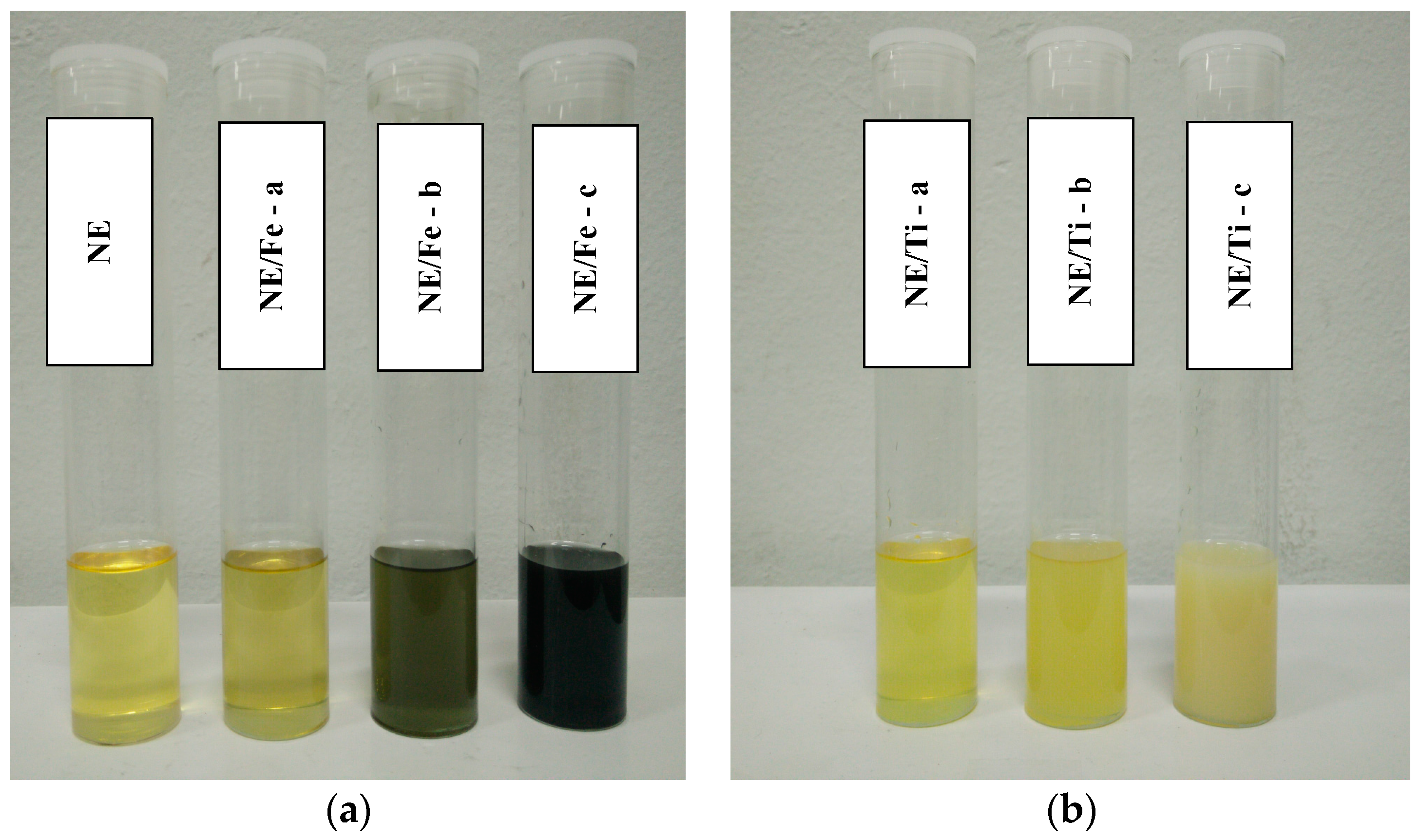
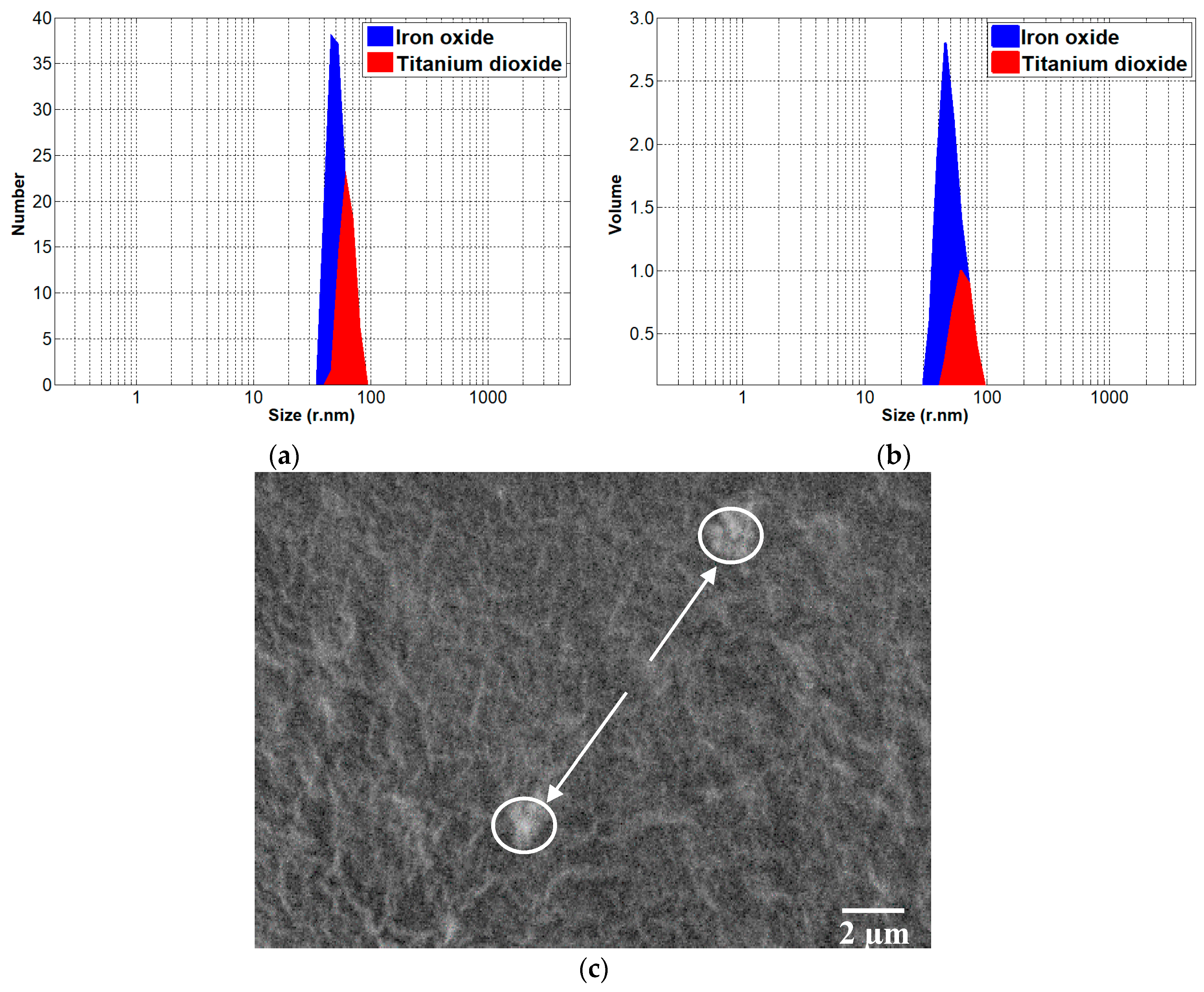
| Sample Abbreviation ** | Amount of Nanoparticles [g/L] | |
|---|---|---|
| Iron Oxide | Titanium Dioxide | |
| NE | - | - |
| NE/Fe-a | 0.01 | - |
| NE/Fe-b | 0.1 | - |
| NE/Fe-c | 1.0 | |
| NE/Ti-a | - | 0.01 |
| NE/Ti-b | - | 0.1 |
| NE/Ti-c | - | 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makmud, M.Z.H.; Illias, H.A.; Chee, C.Y.; Sarjadi, M.S. Influence of Conductive and Semi-Conductive Nanoparticles on the Dielectric Response of Natural Ester-Based Nanofluid Insulation. Energies 2018, 11, 333. https://doi.org/10.3390/en11020333
Makmud MZH, Illias HA, Chee CY, Sarjadi MS. Influence of Conductive and Semi-Conductive Nanoparticles on the Dielectric Response of Natural Ester-Based Nanofluid Insulation. Energies. 2018; 11(2):333. https://doi.org/10.3390/en11020333
Chicago/Turabian StyleMakmud, M. Z. H., H. A. Illias, C. Y. Chee, and M. S. Sarjadi. 2018. "Influence of Conductive and Semi-Conductive Nanoparticles on the Dielectric Response of Natural Ester-Based Nanofluid Insulation" Energies 11, no. 2: 333. https://doi.org/10.3390/en11020333
APA StyleMakmud, M. Z. H., Illias, H. A., Chee, C. Y., & Sarjadi, M. S. (2018). Influence of Conductive and Semi-Conductive Nanoparticles on the Dielectric Response of Natural Ester-Based Nanofluid Insulation. Energies, 11(2), 333. https://doi.org/10.3390/en11020333






