Comparison of Cassava Starch with Corn as a Feedstock for Bioethanol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
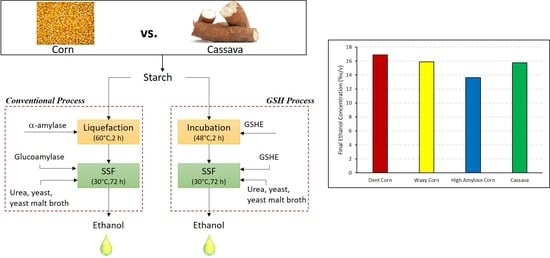
2.2. Conventional Process
2.3. Granular Starch Hydrolysis (GSH)
2.4. HPLC analysis
2.5. Fermentation Rates and Ethanol Yields
2.6. Statistical Analysis
3. Results
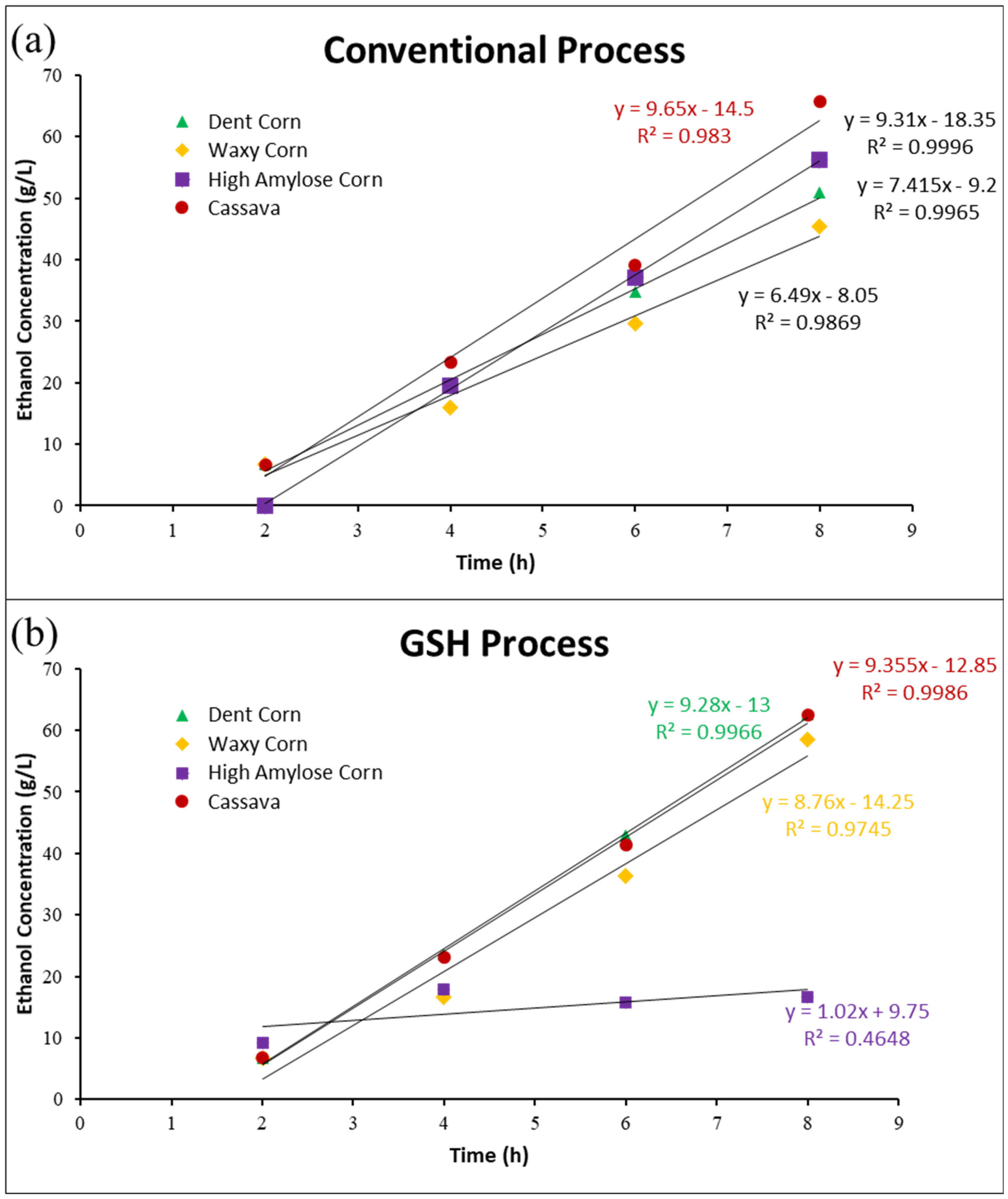
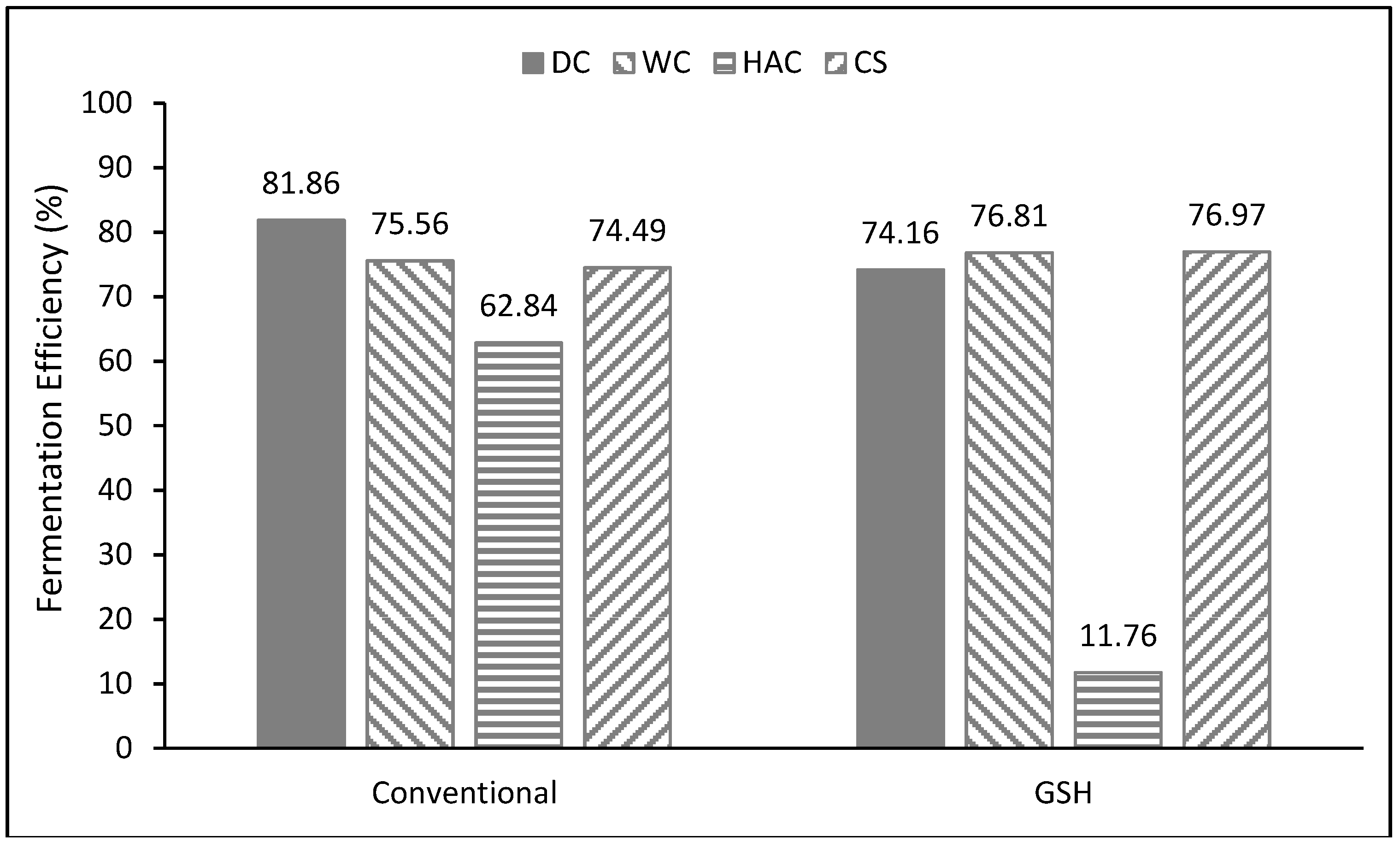
3.1. Conventional Dry Grind Process
3.2. GSH Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Renewable Fuel Association. Ethanol Industry Outlook. 2017. Available online: http://www.ethanolrfa.org/resources/industry/statistics/#1454098996479-8715d404-e546 (accessed on 16 June 2018).
- Olguin-Maciel, E.; Larqué-Saavedra, A.; Pérez-Brito, D.; Barahona-Pérez, L.F.; Alzate-Gaviria, L.; Toledano-Thompson, T.; Lappe-Oliveras, P.E.; Huchin-Poot, E.G.; Tapia-Tussell, R. Brosimum Alicastrum as a Novel Starch Source for Bioethanol Production. Energies 2017, 10, 1574. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Medeiros, A.B.P.; Rodrigues, C.; de Souza Vandenberghe, L.P.; de Andrade Tanobe, V.O.; Dall’Agnol, A.; Gazzoni, D.L.; Soccol, C.R. Feedstocks for Biofuels. In Green Fuels Technology; Soccol, C.R., Brar, S.K., Faulds, C., Ramos, L.P., Eds.; Springer International Publishing: AG, Switzerland, 2016; pp. 15–39. [Google Scholar]
- Okudoh, V.; Trois, C.; Workneh, T.; Schmidt, S. The potential of cassava biomass and applicable technologies for sustainable biogas production in South Africa: A review. Renew. Sustain. Energy Rev. 2014, 39, 1035–1052. [Google Scholar] [CrossRef]
- Sebayang, A.H.; Hassan, M.H.; Ong, H.C.; Dharma, S.; Silitonga, A.S.; Kusumo, F.; Mahlia, T.M.I.; Bahar, A.H. Optimization of Reducing Sugar Production from Manihot glaziovii Starch Using Response Surface Methodology. Energies 2017, 10, 35. [Google Scholar] [CrossRef]
- Moshi, A.P.; Hosea, K.M.; Elisante, E.; Mamo, G.; Mattiasson, B. High temperature simultaneous saccharification and fermentation of starch from inedible wild cassava (Manihot glaziovii) to bioethanol using Caloramator boliviensis. Bioresource Technol. 2015, 180, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Pervez, S.; Aman, A.; Iqbal, S.; Siddiqui, N.N.; Qader, S.A.U. Saccharification and liquefaction of cassava starch: An alternative source for the production of bioethanol using amylolytic enzymes by double fermentation process. BMC Biotechnol. 2014, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.V.S.; Basappa, S. Direct fermentation of cassava starch to ethanol by mixed cultures of Endomycopsis fibuligera and Zymomonas mobilis: Synergism and limitations. Biotechnol. Lett. 1996, 18, 1315–1318. [Google Scholar] [CrossRef]
- Ueda, S.; Zenin, C.T.; Monteiro, D.A.; Park, Y.K. Production of ethanol from raw cassava starch by a nonconventional fermentation method. Biotechnol. Bioeng. 1981, 23, 291–299. [Google Scholar] [CrossRef]
- Kurambhatti, C.V.; Kumar, D.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Ethanol production from corn fiber separated after liquefaction in the dry grind process. Energies 2018, 11, 2921. [Google Scholar] [CrossRef]
- Sriroth, K.; Wanlapatit, S.; Piyachomkwan, K. Cassava bioethanol. In Bioethanol; Lima, M.A.P., Ed.; InTechOpen Publishing: London, UK, 2012; pp. 3–32. [Google Scholar]
- Wang, W. Cassava production for industrial utilization in China–present and future perspective. In Proceedings of the Cassava research and development in Asia: Exploring New Opportunities for an Ancient Crop: Seventh Regional Cassava Workshop, Bangkok, Thailand, 28 October–1 November 2002; pp. 33–38. [Google Scholar]
- Khamkeaw, A.; Phisalaphong, M. Hydrolysis of cassava starch by co-immobilized multi-microorganisms of Loog-Pang (Thai rice cake starter) for ethanol fermentation. Food Sci. Biotechnol. 2016, 25, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Yuangsaard, N.; Yongmanitchai, W.; Yamada, M.; Limtong, S. Selection and characterization of a newly isolated thermotolerant Pichia kudriavzevii strain for ethanol production at high temperature from cassava starch hydrolysate. Antonie Van Leeuwenhoek 2013, 103, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.C. Starch in Food: Structure, Function and Applications; Woodhead Publishing Limited: Oxford, UK, 2004. [Google Scholar]
- Wu, X.; Zhao, R.; Wang, D.; Bean, S.; Seib, P.; Tuinstra, M.; Campbell, M.; O’brien, A. Effects of amylose, corn protein, and corn fiber contents on production of ethanol from starch-rich media. Cereal Chem. 2006, 83, 569–575. [Google Scholar] [CrossRef]
- Breuninger, W.F.; Piyachomkwan, K.; Sriroth, K. Tapioca/cassava starch: Production and use. In Starch, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 541–568. [Google Scholar]
- Sharma, V.; Rausch, K.D.; Graeber, J.V.; Schmidt, S.J.; Buriak, P.; Tumbleson, M.; Singh, V. Effect of resistant starch on hydrolysis and fermentation of corn starch for ethanol. Appl. Biochem. Biotechnol. 2010, 160, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Singh, V.; Xue, H.; Johnston, D.B.; Rausch, K.D.; Tumbleson, M.E. Comparison of raw starch hydrolyzing enzyme with conventional liquefaction and saccharification enzymes in dry-grind corn processing. Cereal Chem. 2007, 84, 10–14. [Google Scholar] [CrossRef]
- Kumar, D.; Moy, K.; Singh, V. Ethanol yield calculation method–“an unaccounted factor” responsible for yield variations. In Proceedings of the 2017 ASABE Annual International Meeting, Spokane, WA, USA, 16–19 July 2017; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA; p. 1701287. [Google Scholar]
- Sharma, V.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Comparison between granular starch hydrolyzing enzyme and conventional enzymes for ethanol production from maize starch with different amylose: Amylopectin ratios. Starch-Stärke 2007, 59, 549–556. [Google Scholar] [CrossRef]
- Sriroth, K.; Piyachomkwan, K.; Wanlapatit, S.; Nivitchanyong, S. The promise of a technology revolution in cassava bioethanol: From Thai practice to the world practice. Fuel 2010, 89, 1333–1338. [Google Scholar] [CrossRef]
- Juneja, A.; Kumar, D.; Singh, V. Germ soak water as nutrient source to improve fermentation of corn grits from modified corn dry grind process. Bioresour. Bioprocess. 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Nordström, K. Yeast growth and glycerol formation. Acta Chem. Scand. 1966, 20, 1016–1025. [Google Scholar] [CrossRef] [PubMed]



| Corn Starch | Cassava Starch | |
|---|---|---|
| Content of starch (% db) | 64 to 78 | 30 to 35 |
| Average granular diameter (μm) | 10 | 15 |
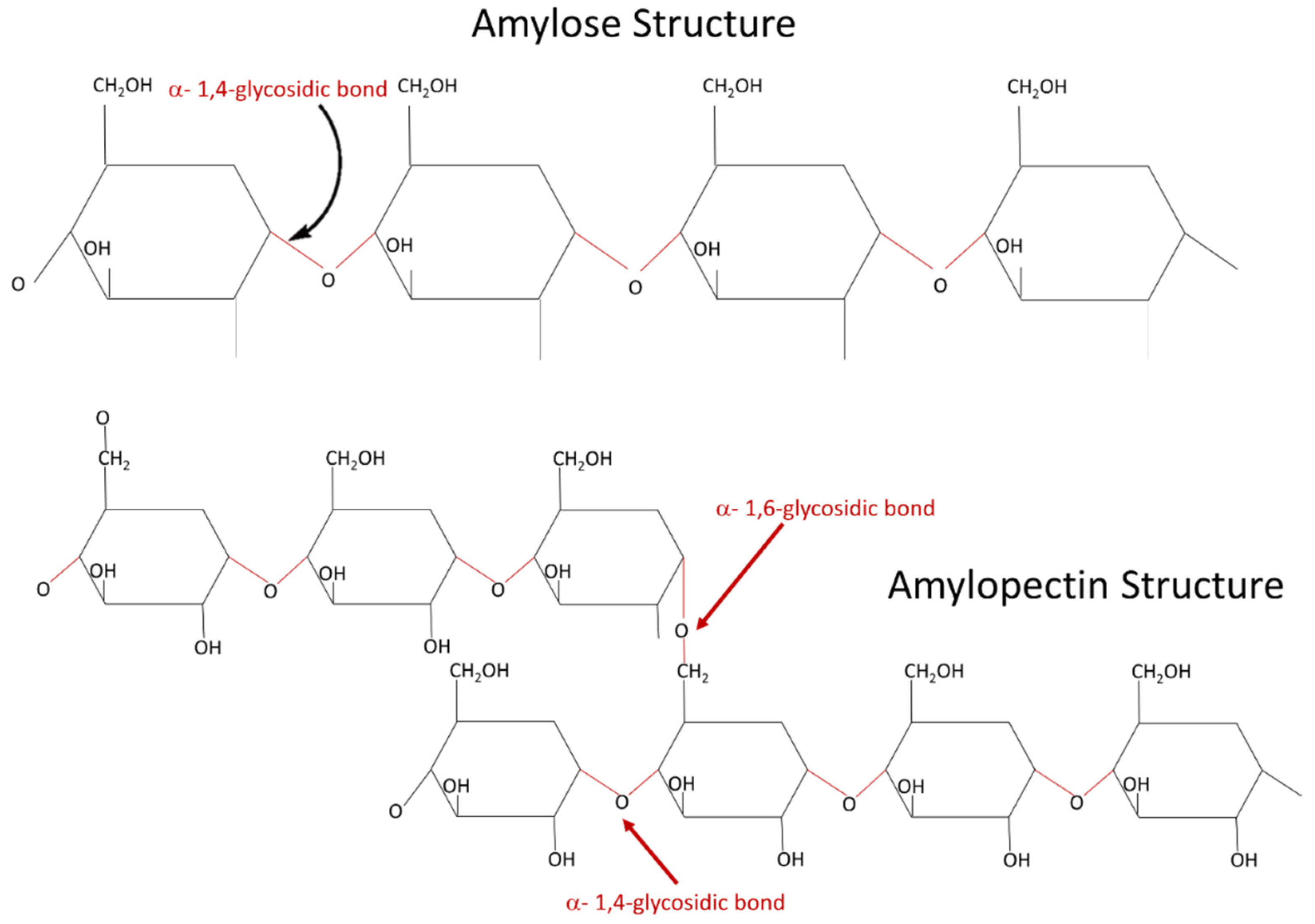
| Amylose content (% of total starch) | 20 to 30 | 17 |
| Degree of polymerization (DPn) | 3000 | 800 |
| Gelatinization temperature range (°C) | 75 to 80 | 65 to 70 |
| Starch Type | Conventional | GSH |
|---|---|---|
| Dent corn | 0.7415 b | 0.9355 a |
| Waxy corn | 0.649 c | 0.876 b |
| High amylose corn | 0.931 a | 0.102 c |
| Cassava | 0.965 a | 0.928 a |
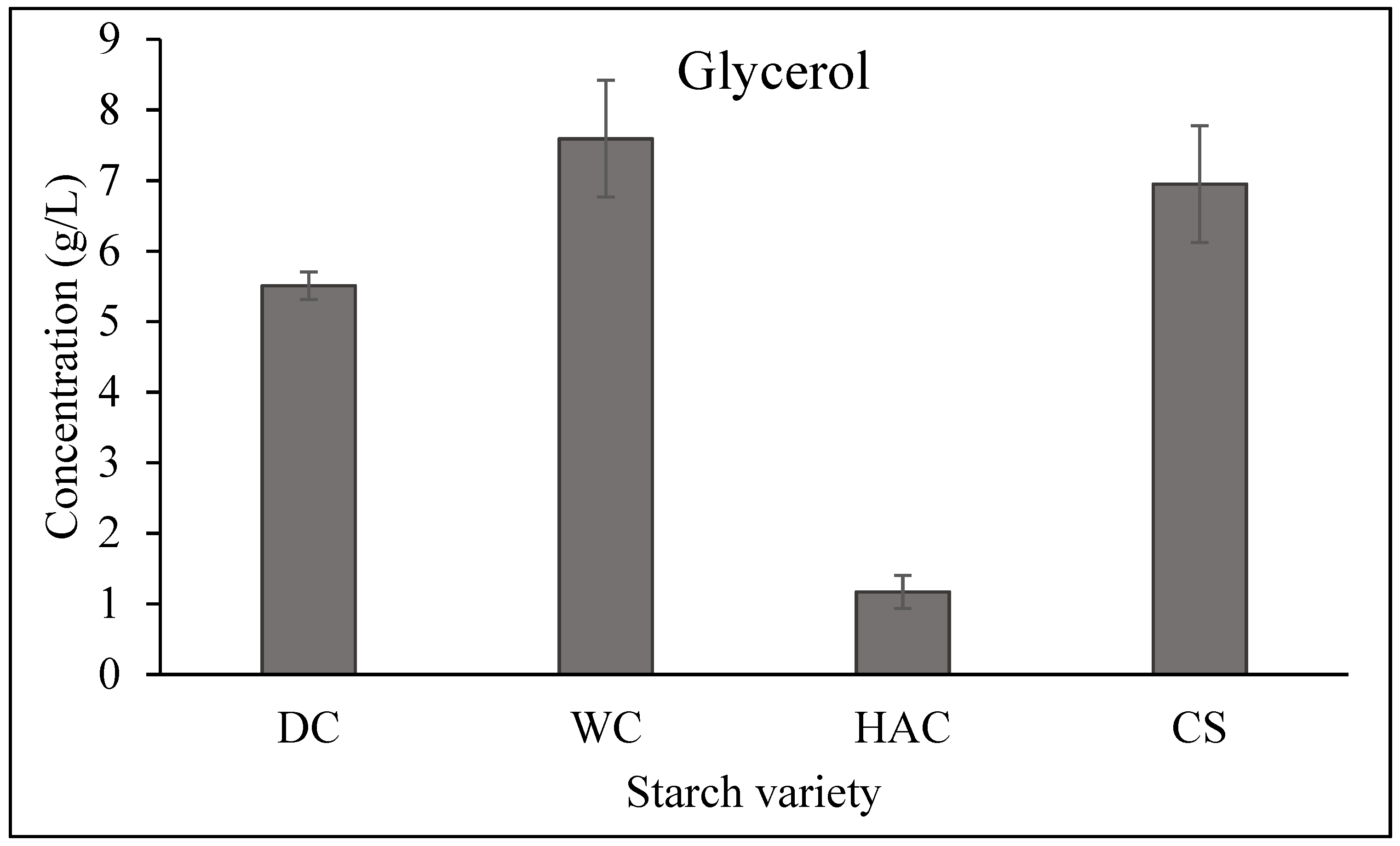
| Time (hours) | Maltotriose (g/L) | Maltose (g/L) | Glycerol (g/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DCS | WCS | HACS | CS | DCS | WCS | HASC | CS | DCS | WCS | HACS | CS | |
| 0 | 30.2 | 22.08 | 33.94 | 29.11 | 32.36 | 28.67 | 0.00 | 31.89 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 36.81 | 16.10 | 1.66 | 10.65 | 72.96 | 82.55 | 0.00 | 94.03 | 0.90 | 0.00 | 0.00 | 0.81 |
| 4 | 21.8 | 2.96 | 0.57 | 2.98 | 90.93 | 85.22 | 0.00 | 93.50 | 1.96 | 0.00 | 1.98 | 1.75 |
| 6 | 11.12 | 0.64 | 0.36 | 1.13 | 80.59 | 82.78 | 0.00 | 92.94 | 2.96 | 0.00 | 3.10 | 2.86 |
| 8 | 9.305 | 0.36 | 0.27 | 0.56 | 96.27 | 76.32 | 0.00 | 86.50 | 3.93 | 0.00 | 3.5 | 4.00 |
| 20 | 0.45 | 0.95 | 0.81 | 0.80 | 47.65 | 30.95 | 0.00 | 43.20 | 5.75 | 4.55 | 4.36 | 5.85 |
| 24 | 0.68 | 1.42 | 0.00 | 1.09 | 35.64 | 19.59 | 0.00 | 30.23 | 6.08 | 4.62 | 4.56 | 5.99 |
| 48 | 1.63 | 1.58 | 0.47 | 2.15 | 1.77 | 0.95 | 0.00 | 1.20 | 7.70 | 5.20 | 5.61 | 6.92 |
| 72 | 0.51 | 0.35 | 0.31 | 0.40 | 0.85 | 0.00 | 0.00 | 0.42 | 8.02 | 5.30 | 5.84 | 7.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradyawong, S.; Juneja, A.; Sadiq, M.B.; Noomhorm, A.; Singh, V. Comparison of Cassava Starch with Corn as a Feedstock for Bioethanol Production. Energies 2018, 11, 3476. https://doi.org/10.3390/en11123476
Pradyawong S, Juneja A, Sadiq MB, Noomhorm A, Singh V. Comparison of Cassava Starch with Corn as a Feedstock for Bioethanol Production. Energies. 2018; 11(12):3476. https://doi.org/10.3390/en11123476
Chicago/Turabian StylePradyawong, Sarocha, Ankita Juneja, Muhammad Bilal Sadiq, Athapol Noomhorm, and Vijay Singh. 2018. "Comparison of Cassava Starch with Corn as a Feedstock for Bioethanol Production" Energies 11, no. 12: 3476. https://doi.org/10.3390/en11123476