Hybrid Density Functional Study of Au2Cs2I6, Ag2GeBaS4, Ag2ZnSnS4, and AgCuPO4 for the Intermediate Band Solar Cells
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Structural Properties
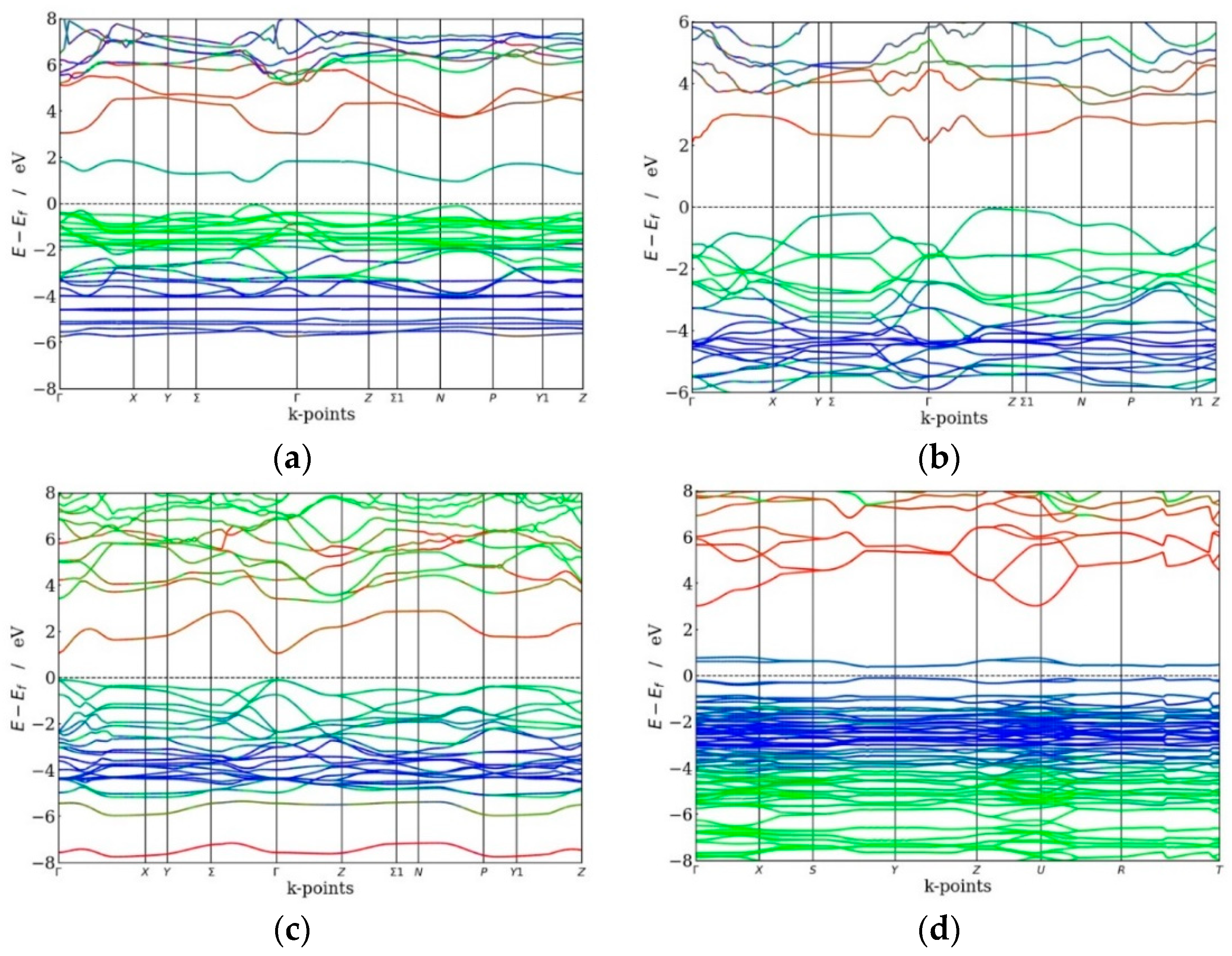
3.2. Electronic Properties
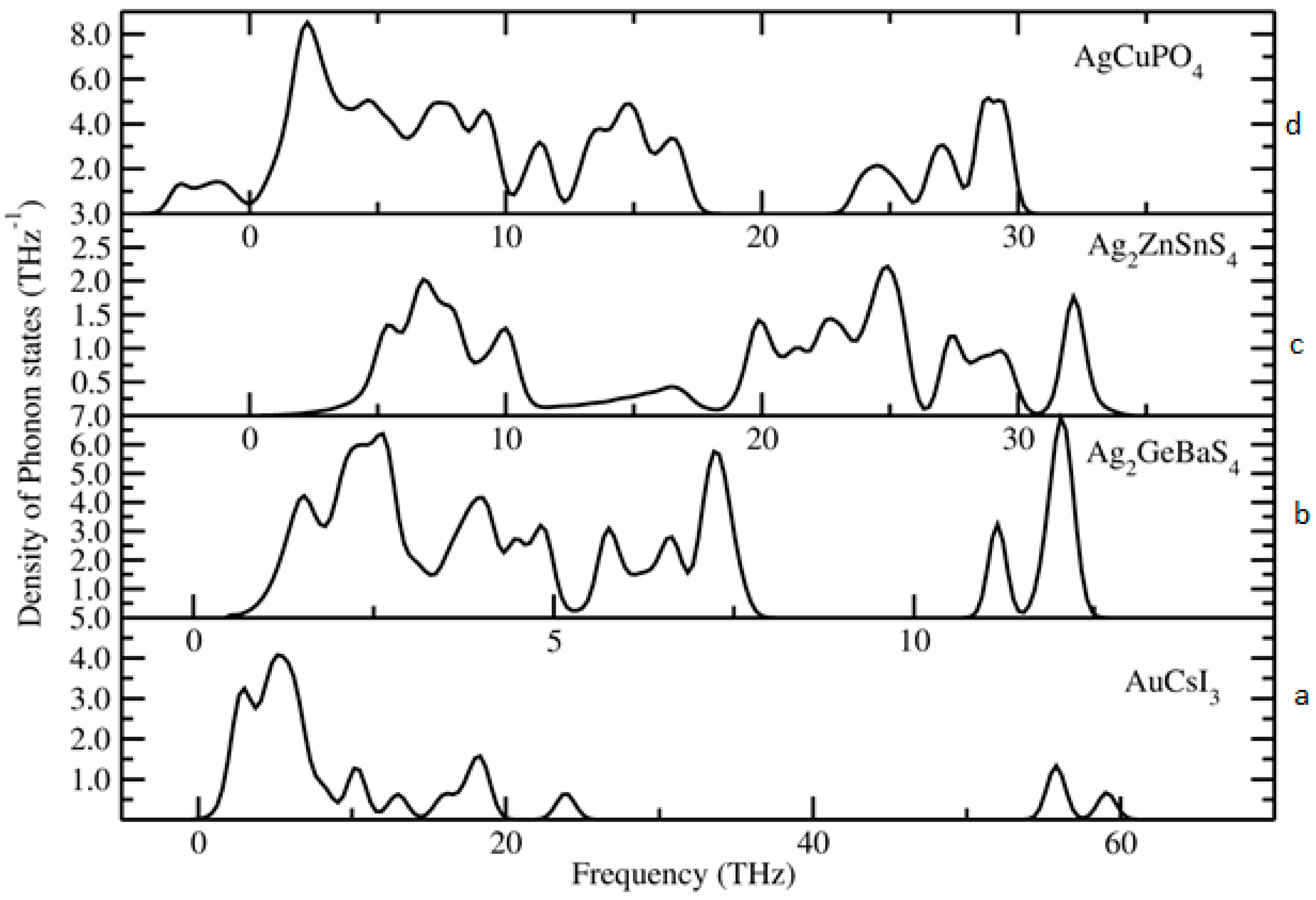
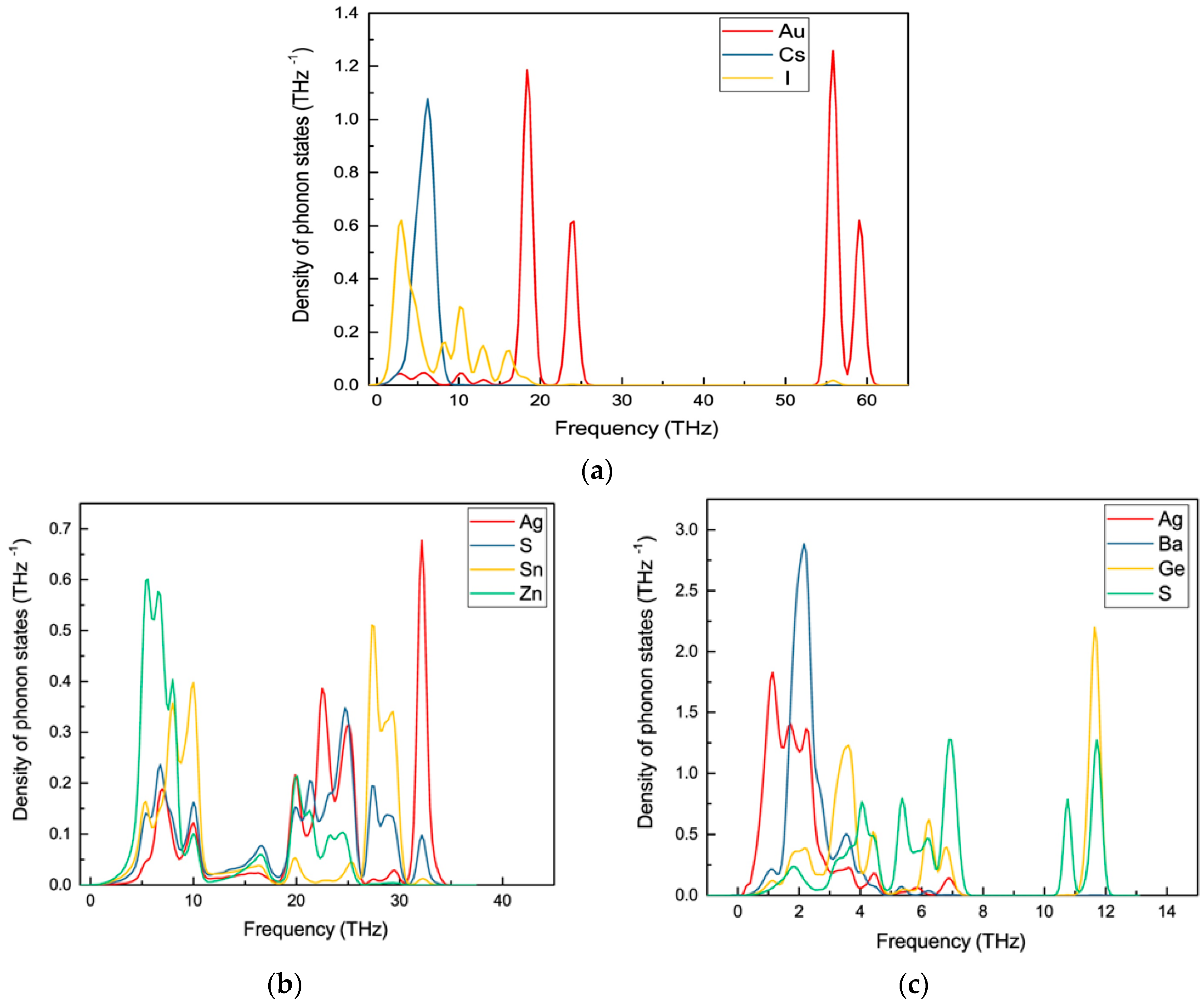
3.3. Lattice Dynamical Stability
3.4. Mechanical Stability
Single Crystal Elastic Constants and Mechanical Stability
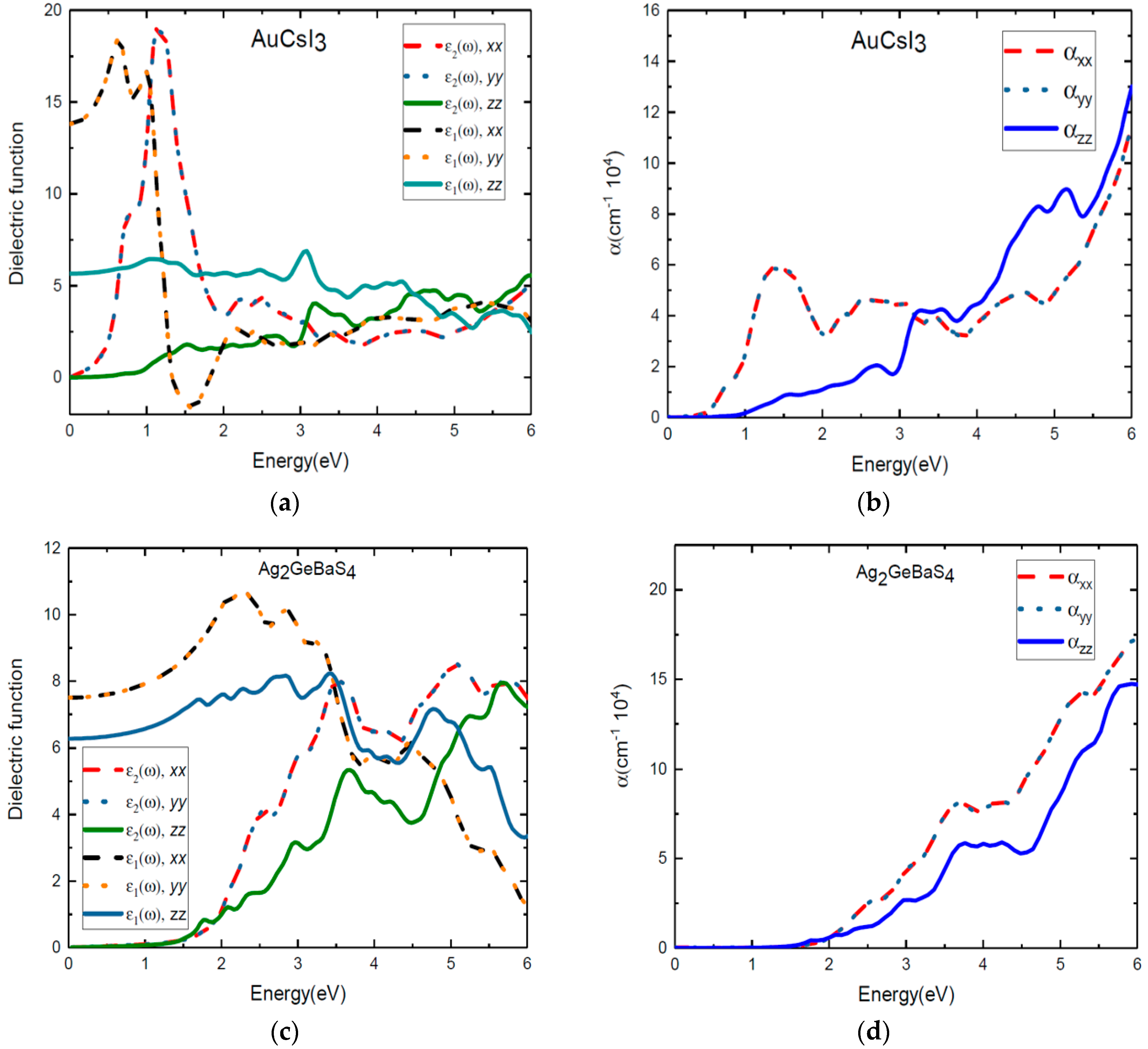
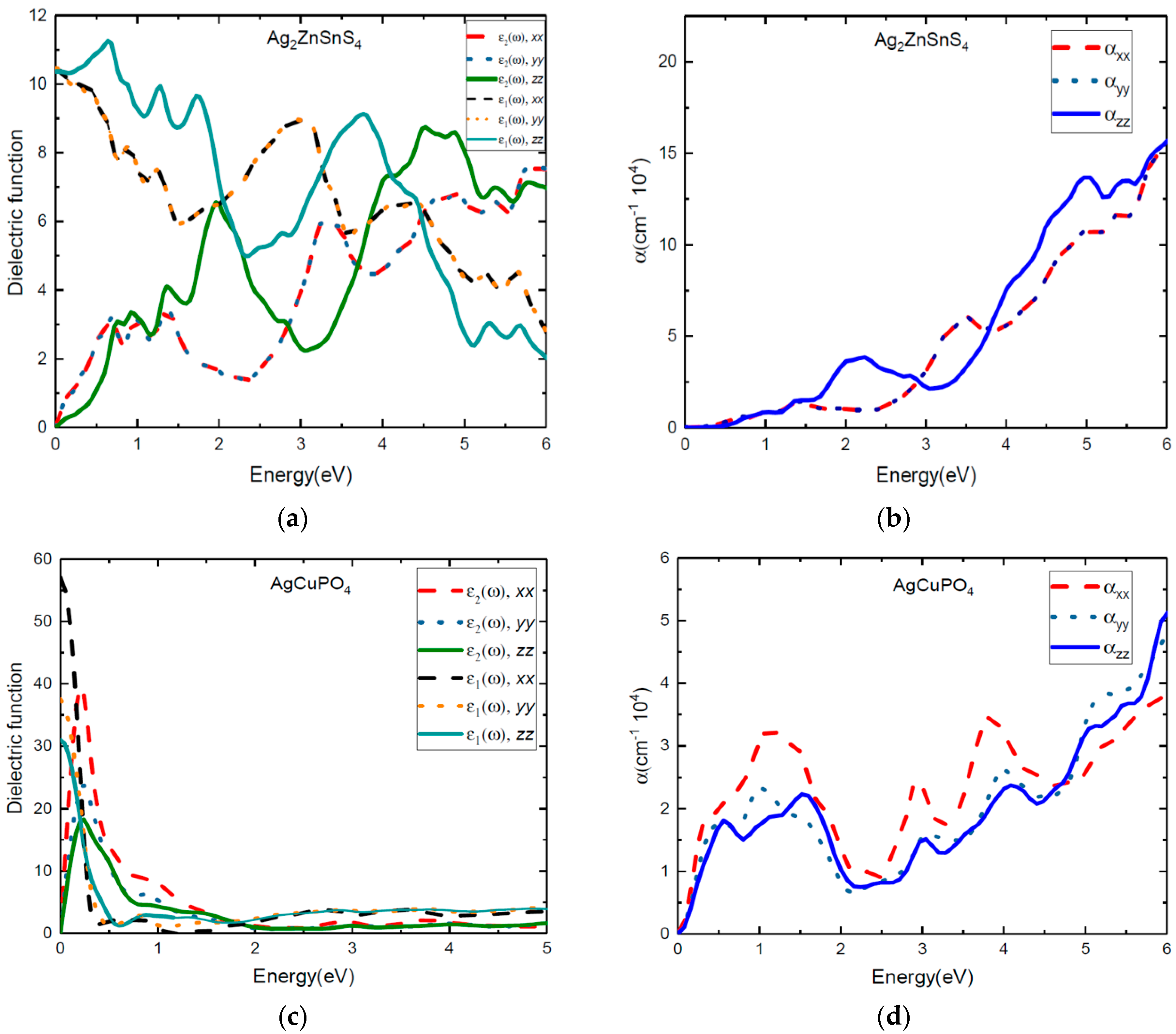
3.5. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasukkannu, M.; Velauthapillai, D.; Vajeeston, P. Computational Modeling of Novel Bulk Materials for the Intermediate-Band Solar Cells. ACS Omega 2017, 2, 1454–1462. [Google Scholar] [CrossRef]
- Luque, A.; Martí, A.; Stanley, C. Understanding intermediate-band solar cells. Nat. Photonics 2012, 6, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Luque, A.; Marti, A. A metallic intermediate band high efficiency solar cell. Prog. Photovolt. Res. Appl. 2001, 9, 73–86. [Google Scholar] [CrossRef]
- Rasukkannu, M.; Velauthapillai, D.; Vajeeston, P. A first-principle study of the electronic, mechanical and optical properties of inorganic perovskite Cs2SnI6 for intermediate-band solar cells. Mater. Lett. 2018, 218, 233–236. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Luque, A.; Martí, A. Increasing the efficiency of ideal solar cells by photon induced transitions at intermediate levels. Phys. Rev. Lett. 1997, 78, 5014. [Google Scholar] [CrossRef]
- Palacios, P.; Aguilera, I.; Sánchez, K.; Conesa, J.; Wahnón, P. Transition-metal-substituted indium thiospinels as novel intermediate-band materials: Prediction and understanding of their electronic properties. Phys. Rev. Lett. 2008, 101, 046403. [Google Scholar] [CrossRef]
- Green, M.A. Multiple band and impurity photovoltaic solar cells: General theory and comparison to tandem cells. Prog. Photovolt. Res. Appl. 2001, 9, 137–144. [Google Scholar] [CrossRef]
- Nozawa, T.; Arakawa, Y. Detailed balance limit of the efficiency of multilevel intermediate band solar cells. Appl. Phys. Lett. 2011, 98, 171108. [Google Scholar] [CrossRef]
- Okada, Y.; Ekins-Daukes, N.; Kita, T.; Tamaki, R.; Yoshida, M.; Pusch, A.; Hess, O.; Phillips, C.; Farrell, D.; Yoshida, K. Intermediate band solar cells: Recent progress and future directions. Appl. Phys. Rev. 2015, 2, 021302. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, I.; Palacios, P.; Wahnón, P. Optical properties of chalcopyrite-type intermediate transition metal band materials from first principles. Thin Solid Films 2008, 516, 7055–7059. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Zhang, X.; Zeng, Z. The investigation of transition metal doped CuGaS2 for promising intermediate band materials. RSC Adv. 2014, 4, 62380–62386. [Google Scholar] [CrossRef]
- Aguilera, I.; Palacios, P.; Wahnón, P. Enhancement of optical absorption in Ga-chalcopyrite-based intermediate-band materials for high efficiency solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1903–1906. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Qin, M.; Chen, H.; Yang, C.; Wang, Y.; Huang, F. Cr incorporation in CuGaS2 chalcopyrite: A new intermediate-band photovoltaic material with wide-spectrum solar absorption. Phys. Status Solidi (a) 2013, 210, 1098–1102. [Google Scholar] [CrossRef]
- Han, M.; Zhang, X.; Zhang, Y.; Zeng, Z. The group VA element non-compensated n–p codoping in CuGaS2 for intermediate band materials. Sol. Energy Mater. Sol. Cells 2016, 144, 664–670. [Google Scholar] [CrossRef]
- Gong, W.; Tabata, T.; Takei, K.; Morihama, M.; Maeda, T.; Wada, T. Crystallographic and optical properties of (Cu, Ag)2ZnSnS4 and (Cu, Ag)2ZnSnSe4 solid solutions. Phys. Status Solidi (c) 2015, 12, 700–703. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Dudarev, S.; Botton, G.; Savrasov, S.Y.; Szotek, Z.; Temmerman, W.; Sutton, A. Electronic Structure and Elastic Properties of Strongly Correlated Metal Oxides from First Principles: LSDA+ U, SIC-LSDA and EELS Study of UO2 and NiO. Phys. Status Solidi (a) 1998, 166, 429–443. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Liechtenstein, A.; Anisimov, V.; Zaanen, J. Density-functional theory and strong interactions: Orbital ordering in Mott-Hubbard insulators. Phys. Rev. B 1995, 52, R5467. [Google Scholar] [CrossRef]
- Ponniah, V. Density Functional Theory Based Database (DFTBD); University of Oslo: Oslo, Norway, 2013. [Google Scholar]
- Heyd, J.; Scuseria, G.E. Efficient hybrid density functional calculations in solids: Assessment of the Heyd–Scuseria–Ernzerhof screened Coulomb hybrid functional. J. Chem. Phys. 2004, 121, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Gajdoš, M.; Hummer, K.; Kresse, G.; Furthmüller, J.; Bechstedt, F. Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B 2006, 73, 045112. [Google Scholar] [CrossRef]
- Paier, J.; Marsman, M.; Kresse, G. Dielectric properties and excitons for extended systems from hybrid functionals. Phys. Rev. B 2008, 78, 121201. [Google Scholar] [CrossRef]
- Hellenbrandt, M. The inorganic crystal structure database (ICSD)—Present and future. Crystallogr. Rev. 2004, 10, 17–22. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Togo, A.; Oba, F.; Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 2008, 78, 134106. [Google Scholar] [CrossRef]
- Matsushita, N.; Kitagawa, H.; Kojima, N. A Three-Dimensional Iodo-Bridged Mixed-Valence Gold (I, III) Compound, Cs2AuIAuIIII6. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 663–666. [Google Scholar] [CrossRef]
- Liu, X.; Matsuda, K.; Moritomo, Y.; Nakamura, A.; Kojima, N. Electronic structure of the gold complexes Cs2Au2X6 (X = I, Br, and Cl). Phys. Rev. B 1999, 59, 7925. [Google Scholar] [CrossRef]
- Teske, C.L. Darstellung und Kristallstruktur von Silber-Barium-Thiogermanat (IV). Ag2BaGeS4/Preparation and Crystal Structure of Silver-Barium-Thiogermanate (IV). Ag2BaGeS4. Zeitschrift für Naturforschung B 1979, 34, 544–547. [Google Scholar] [CrossRef]
- Quarton, M.; Oumba, M. Proprietes de l’ion Cu2+ dans la structure de AgCuPO4-β. Mater. Res. Bull. 1983, 18, 967–974. [Google Scholar] [CrossRef]
- Riggs, S.C.; Shapiro, M.; Corredor, F.; Geballe, T.; Fisher, I.; McCandless, G.T.; Chan, J.Y. Single crystal growth by self-flux method of the mixed valence gold halides Cs2 [AuIX2][AuIIIX4](X = Br, I). J. Cryst. Growth 2012, 355, 13–16. [Google Scholar] [CrossRef]
- Johan, Z.; Picot, P. La pirquitasite, Ag2ZnSnS4, un nouveau membre du groupe de la stannite. Bull. Miner. 1982, 105, 229–235. [Google Scholar]
- Ben Yahia, H.; Gaudin, E.; Darriet, J.; Dai, D.; Whangbo, M.-H. Comparison of the crystal structures and magnetic properties of the low-and high-temperature forms of AgCuPO4: Crystal structure determination, magnetic susceptibility measurements, and spin dimer analysis. Inorg. Chem. 2006, 45, 5501–5509. [Google Scholar] [CrossRef] [PubMed]
- Vajeeston, P.; Fjellvåg, H. First-principles study of structural stability, dynamical and mechanical properties of Li2FeSiO4 polymorphs. RSC Adv. 2017, 7, 16843–16853. [Google Scholar] [CrossRef]
- Hase, M.; Matsuda, M.; Kakurai, K.; Ozawa, K.; Kitazawa, H.; Tsujii, N.; Dönni, A.; Kuroe, H. Inelastic neutron scattering study of the spin-gap cuprate β−AgCuPO4. Phys. Rev. B 2007, 76, 134403. [Google Scholar] [CrossRef]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics (Holt, Rinehart and Winston, New York, 1976). Google Sch. 2005, 403, 444–447. [Google Scholar]
- Nye, J.F. Physical Properties of Crystals: Their Representation by Tensors and Matrices; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Reuss, A. Berechnung der fließgrenze von mischkristallen auf grund der plastizitätsbedingung für einkristalle. ZAMM-J. Appl. Math. Mech./Zeitschrift für Angewandte Mathematik und Mechanik 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Pugh, S. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Yang, L.-M.; Vajeeston, P.; Ravindran, P.; Fjellvåg, H.; Tilset, M. Revisiting isoreticular MOFs of alkaline earth metals: A comprehensive study on phase stability, electronic structure, chemical bonding, and optical properties of A–IRMOF-1 (A = Be, Mg, Ca, Sr, Ba). Phys. Chem. Chem. Phys. 2011, 13, 10191–10203. [Google Scholar] [CrossRef] [PubMed]
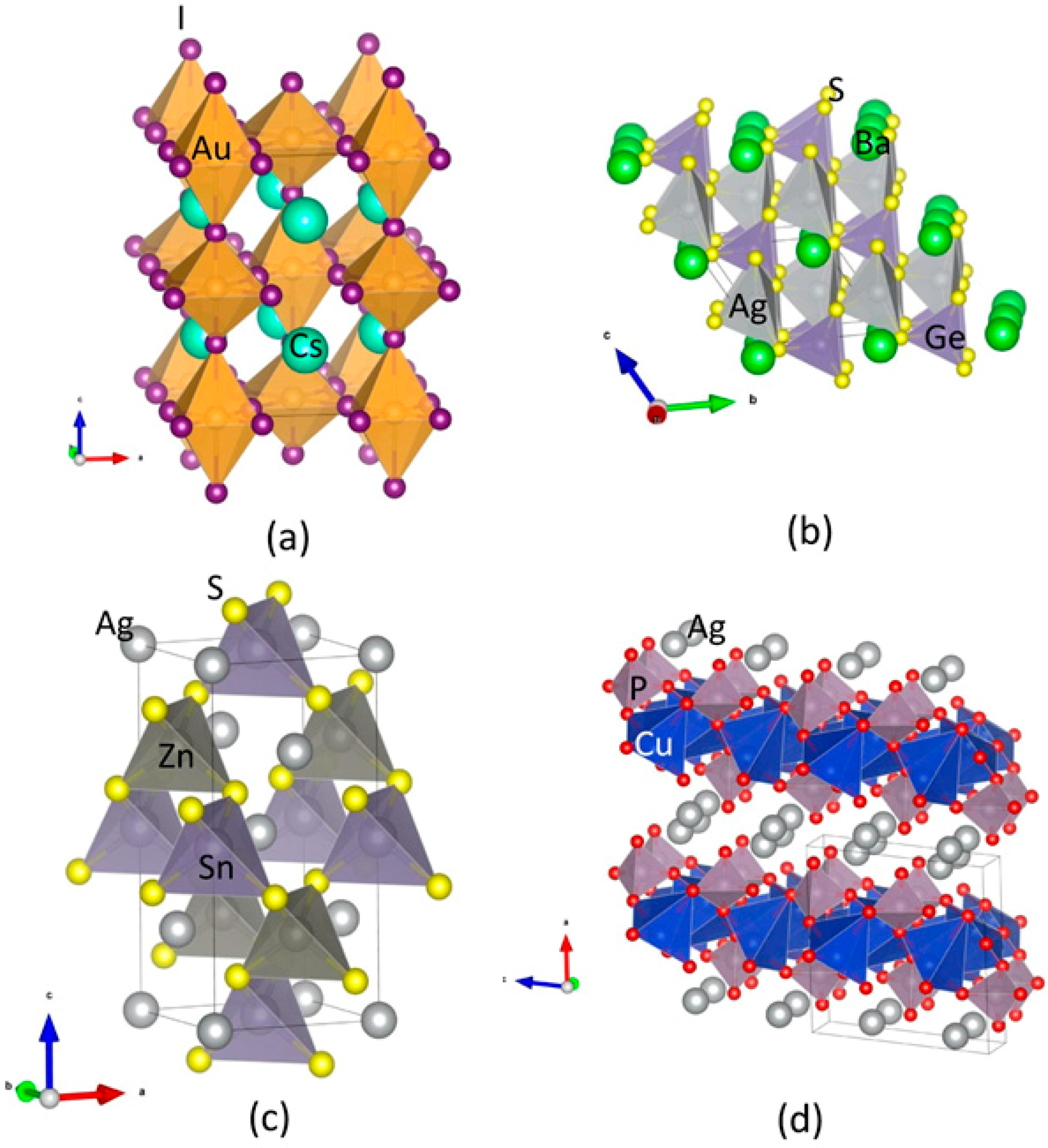

| Phase | Lattice Parameter | Atomic Positions | |||
|---|---|---|---|---|---|
| a | b | c | β(deg) | ||
| Au2Cs2I6- I4/mmm; 139 | 8.4089 (8.2847 c) | 12.301 (12.0845 c) | 90 | c Cs(4d): 0, 1/2, 1/4 c Au1(2a): 0, 0, 0 c Au2(2b):1/2, 1/2, 0 c I1(8h):0.2258, 0.2285, 0 c I2(4e):1/2, 1/2, 0.2131 | |
| Ag2BaGeS4- I-42m 121 | 6.9327 (6.8280 b) | 8.1705 (8.0170 b) | 90 | b Ba(2a): 0, 0, 0 b Ag(4d): 0, 1/2, 1/4 b Ge(2b): 0, 0, 1/2 b S(8i): 0.1883, 0.1883, 0.3440 | |
| Ag2ZnSnS4- I-4m; 121 | 5.703 (5.693 d) | 11.350 (11.342 d) | 90 | d Zn(2d): 1/2, 0, 1/4 d Sn(2b): 1/2, 1/2, 0 d Ag(2c): 0, 1/2, 1/4 d Ag(2a): 0, 0, 0 d S(8g):0.249, 0.240, 0.128 | |
| Ag2ZnSnS4- I-42m | 5.6503 (5.7860 e) | 11.4884 (10.8290 e) | 90 | e Zn(2a): 0, 0, 0 e Sn(2b): 1/2, 1/2, 0 e Ag(4d): 0, 1/2, 1/4 e S(8i):0.7560, 0.7560, 0.8700 | |
| AgCuPO4- P21/c; 14 | 8.010 (7.8365 a) | 5.6438 (5.6269 a) | 7.6480 (7.4938 a) | 98.15 (99.07 a) | a Ag(4e): 0.9287, 0.1093, 0.1909 a Cu(4e): 0.5834, 0.1205, 0.8533 a P(4e): 0.2754, 0.1150, 0.4925 a O1(4e): 0.3150, −0.0490, 0.6480 a O2(4e): 0.2760, −0.0130, 0.3120 a O3(4e): 0.0990, 0.2370, 0.4920 a O4(4e): 0.4280, 0.3050, 0.5200 |
| Compounds | Bandgap (Evi) | Bandgap (Eci) | Width of IB (ΔEi) | Total Bandgap (Eg) |
|---|---|---|---|---|
| Au2Cs2I6 | 0.89 | 1.13 | 0.90 | 2.92 |
| Ag2GeBaS4 | 2.08 | 0.34 | 0.91 | 3.33 |
| Ag2ZnSnS4 | 1.15 | 0.34 | 1.85 | 3.34 |
| AgCuPO4 | 0.33 | 2.19 | 0.44 | 2.96 |
| Properties | Phase | ||||
|---|---|---|---|---|---|
| AgCuPO4 | Ag2GeBaS4 | Au2Cs2I6 | Ag2ZnSnS4 | ||
| P21/n | I-42m | I4/mmm | I-4m | I-42m | |
| Cij | C11 = 86 | C11 = 74 | C11 = 18 | C11 = 67 | C11 = 63 |
| C12 = 66 | C12 = 48 | C12 = 11 | C12 = 44 | C12 = 45 | |
| C13 = 52 | C13 = 33 | C13 =3 | C13 = 43 | C13 = 44 | |
| C16 = 18 | C33 = 47 | C33 = 21 | C33 = 63 | C33 = 74 | |
| C22 = 162 | C44 = 22 | C44 = 7 | C44 = 28 | C44 = 29 | |
| C23 = 72 | C55 = 18 | C55 = 1 | C55 = 25 | C55 = 27 | |
| C26 = 9 | |||||
| C33 = 110 | |||||
| C36 = 23 | |||||
| C44 = 21 | |||||
| C45 = 5 | |||||
| C55 = 18 | |||||
| C66 = 19 | |||||
| BV | 82 | 47 | 10 | 51 | 52 |
| BR | 52 | 43 | 10 | 51 | 52 |
| BVRH | 67 | 44.5 | 10 | 51 | 52 |
| GV | 27 | 17 | 4.5 | 20 | 21 |
| GR | 20 | 16 | 2.3 | 17 | 17 |
| GVRH | 23 | 16.7 | 3.4 | 18.3 | 18.5 |
| E | 63 | 45 | 9 | 49 | 50 |
| Compressibility | 0.02 | 0.02 | 0.1 | 0.02 | 0.02 |
| Ductility | 0.35 | 0.38 | 0.34 | 0.36 | 0.36 |
| Lame constant | 51.4 | 33 | 8 | 39 | 39 |
| σ | 0.34 | 0.33 | 0.35 | 0.34 | 0.34 |
| νL | 4366 | 3777 | 1632 | 4073 | 4086 |
| νT | 2129 | 1888 | 792 | 2007 | 2011 |
| ῡ | 2391 | 2118 | 891 | 2253 | 2258 |
| θD | 771 | 592 | 150 | 634 | 637 |
| Compounds | GGA | HSE | Supercell Size | ZPE |
|---|---|---|---|---|
| Au2Cs2I6 | 14.04 | 11.09 | 3 × 3 × 3 | 0.3 |
| Ag2GeBaS4 | 7.48 | 7.09 | 3 × 3 × 2 | 0.24 |
| Ag2ZnSnS4 | 10.45 | 10.42 | 4 × 4 × 2 | 0.25 |
| AgCuPO4 | 64.86 | 41.84 | 2 × 1 × 2 | 0.84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasukkannu, M.; Velauthapillai, D.; Vajeeston, P. Hybrid Density Functional Study of Au2Cs2I6, Ag2GeBaS4, Ag2ZnSnS4, and AgCuPO4 for the Intermediate Band Solar Cells. Energies 2018, 11, 3457. https://doi.org/10.3390/en11123457
Rasukkannu M, Velauthapillai D, Vajeeston P. Hybrid Density Functional Study of Au2Cs2I6, Ag2GeBaS4, Ag2ZnSnS4, and AgCuPO4 for the Intermediate Band Solar Cells. Energies. 2018; 11(12):3457. https://doi.org/10.3390/en11123457
Chicago/Turabian StyleRasukkannu, Murugesan, Dhayalan Velauthapillai, and Ponniah Vajeeston. 2018. "Hybrid Density Functional Study of Au2Cs2I6, Ag2GeBaS4, Ag2ZnSnS4, and AgCuPO4 for the Intermediate Band Solar Cells" Energies 11, no. 12: 3457. https://doi.org/10.3390/en11123457





