Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure
Abstract
:1. Introduction
2. Thermodynamic Modeling of CO2-N2-O2-Brine Equilibria
2.1. Fugacity Coefficients
2.2. Equilibrium Constant
2.3. Activity Model
2.4. Gas-Brine Mutual Solubility Reproduction
3. Gas-Brine-Mineral Equilibria
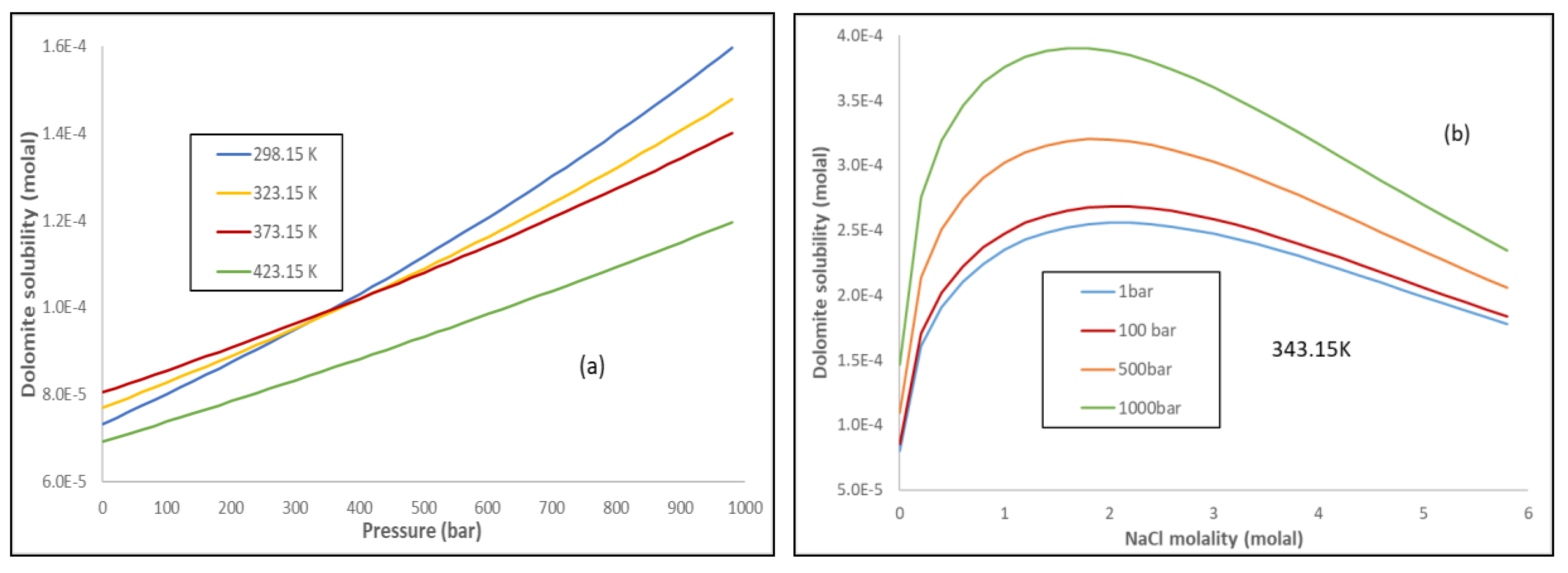
3.1. Mineral Solubility
3.2. Mutual Effects of Dissolutions of Gases and Minerals
3.3. Impurity Effects on Gas-Water-Mineral Equilibria
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, J.; Zhang, K.; Hu, L.; Wang, Y.; Chen, M. Understanding the carbon dioxide sequestration in low-permeability saline aquifers in the Ordos basin with numerical simulations. Greenh. Gases 2015, 5, 558–576. [Google Scholar] [CrossRef]
- IEAGHG. Effects of Impurities on Geological Storage of CO2; Global CCS Institute: Melbourne, Australia, 2011. [Google Scholar]
- Li, J.; Wei, L.; Li, X. An improved cubic model for the mutual solubilities of CO2–CH4–H2S–brine systems to high temperature, pressure and salinity. Appl. Geochem. 2015, 54, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Luo, X.; Li, X. Modelling of phase equilibria in CH4–C2H6–C3H8–nC4H10–NaCl–H2O systems. Appl. Geochem. 2015, 56, 23–36. [Google Scholar] [CrossRef]
- Pearce, J.K.; Dawson, G.K.W.; Law, A.C.K.; Biddle, D.; Golding, S.D. Reactivity of micas and cap-rock in wet supercritical CO2 with SO2 and O2 at CO2 storage conditions. Appl. Geochem. 2016, 72, 59–76. [Google Scholar] [CrossRef]
- Pearce, J.K.; Golab, A.; Dawson, G.K.W.; Knuefing, L.; Goodwin, C.; Golding, S.D. Mineralogical controls on porosity and water chemistry during O2-SO2-CO2 reaction of CO2 storage reservoir and cap-rock core. Appl. Geochem. 2016, 75, 152–168. [Google Scholar] [CrossRef]
- Søreide, I.; Whitson, C.H. Peng-Robinson predictions for hydrocarbons, CO2, N2, and H2S with pure water and NaCl brine. Fluid Phase Equilib. 1992, 77, 217–240. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Mao, S.; Duan, Z. A thermodynamic model for calculating nitrogen solubility, gas phase composition and density of the N2–H2O–NaCl system. Fluid Phase Equilib. 2006, 248, 103–114. [Google Scholar] [CrossRef]
- Geng, M.; Duan, Z. Prediction of oxygen solubility in pure water and brines up to high temperatures and pressures. Geochim. Cosmochim. Acta 2010, 74, 5631–5640. [Google Scholar] [CrossRef]
- Tan, S.P.; Yao, Y.; Piri, M. Modeling the solubility of SO2 + CO2 mixtures in brine at elevated pressures and temperatures. Ind. Eng. Chem. Res. 2013, 52, 10864–10872. [Google Scholar] [CrossRef]
- Sun, R.; Dubessy, J. Prediction of vapor–liquid equilibrium and PVTx properties of geological fluid system with SAFT-LJ EOS including multi-polar contribution. Part I: Application to H2O–CO2 system. Geochim. Cosmochim. Acta 2010, 74, 1982–1998. [Google Scholar] [CrossRef]
- Sun, R.; Dubessy, J. Prediction of vapor–liquid equilibrium and PVTx properties of geological fluid system with SAFT-LJ EOS including multi-polar contribution. Part II: Application to H2O–NaCl and CO2–H2O–NaCl system. Geochim. Cosmochim. Acta 2012, 88, 130–145. [Google Scholar] [CrossRef]
- Sun, R.; Lai, S.; Dubessy, J. Calculation of vapor–liquid equilibrium and PVTx properties of geological fluid system with SAFT-LJ EOS including multi-polar contribution. Part III. Extension to water–light hydrocarbons systems. Geochim. Cosmochim. Acta 2014, 125, 504–518. [Google Scholar] [CrossRef]
- Harvie, C.E.; Weare, J.H. The prediction of mineral solubilities in natural waters: The Na-K-Mg-Ca-Cl-SO4-H2O system from zero to high concentration at 25 °C. Geochim. Cosmochim. Acta 1980, 44, 981–997. [Google Scholar] [CrossRef]
- Harvie, C.E.; Møller, N.; Weare, J.H. The prediction of mineral solubilities in natural waters: The Na-K-Mg-Ca-H-Cl-SO4-OH-HCO3-CO3-CO2-H2O system to high ionic strengths at 25 °C. Geochim. Cosmochim. Acta 1984, 48, 723–751. [Google Scholar] [CrossRef]
- Møller, N. The prediction of mineral solubilities in natural waters: A chemical equilibrium model for the Na-Ca-Cl-SO4-H2O system, to high temperature and concentration. Geochim. Cosmochim. Acta 1988, 52, 821–837. [Google Scholar] [CrossRef]
- Greenberg, J.P.; Møller, N. The prediction of mineral solubilities in natural waters: A chemical equilibrium model for the Na-K-Ca-Cl-SO4-H2O system to high concentration from 0 to 250 °C. Geochim. Cosmochim. Acta 1989, 53, 2503–2518. [Google Scholar] [CrossRef]
- Christov, C.; Moller, N. Chemical equilibrium model of solution behavior and solubility in the H-Na-K-OH-Cl-HSO4-SO4-H2O system to high concentration and temperature. Geochim. Cosmochim. Acta 2004, 68, 1309–1331. [Google Scholar] [CrossRef]
- Duan, Z.; Li, D. Coupled phase and aqueous species equilibrium of the H2O–CO2–NaCl–CaCO3 system from 0 to 250 °C, 1 to 1000 bar with NaCl concentrations up to saturation of halite. Geochim. Cosmochim. Acta 2008, 72, 5128–5145. [Google Scholar] [CrossRef]
- Li, J.; Duan, Z. A thermodynamic model for the prediction of phase equilibria and speciation in the H2O–CO2–NaCl–CaCO3–CaSO4 system from 0 to 250 °C, 1 to 1000 bar with NaCl concentrations up to halite saturation. Geochim. Cosmochim. Acta 2011, 75, 4351–4376. [Google Scholar] [CrossRef]
- Peng, D.-Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Pitzer, K.S. Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 1973, 77, 268–277. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N.S. On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Soave, G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Parkhurst, D.L.; Post, V.E.A. Equations for calculating hydrogeochemical reactions of minerals and gases such as CO2 at high pressures and temperatures. Geochim. Cosmochim. Acta 2014, 125, 49–67. [Google Scholar] [CrossRef]
- Ziabakhsh-Ganji, Z.; Kooi, H. An equation of state for thermodynamic equilibrium of gas mixtures and brines to allow simulation of the effects of impurities in subsurface CO2 storage. Int. J. Greenh. Gas Control 2012, 11, S21–S34. [Google Scholar] [CrossRef]
- Wang, J.; Ryan, D.; Anthony, E.J.; Wigston, A. Effects of impurities on geological storage of carbon dioxide. In Proceedings of the 1st EAGE Sustainable Earth Sciences (SES) Conference and Exhibition, Valencia, Spain, 8–11 November 2011. [Google Scholar]
- Li, H.; Yan, J. Evaluating cubic equations of state for calculation of vapor–liquid equilibrium of CO2 and CO2-mixtures for CO2 capture and storage processes. Appl. Energy 2009, 86, 826–836. [Google Scholar] [CrossRef]
- Appelo, C.A.J. Principles, caveats and improvements in databases for calculating hydrogeochemical reactions in saline waters from 0 to 200 °C and 1 to 1000 atm. Appl. Geochem. 2015, 55, 62–71. [Google Scholar] [CrossRef]
- Helgeson, H.C.; Kirkham, D.H.; Flowers, G.C. Theoretical prediction of the thermodynamic behavior of aqueous electrolytes at high pressures and temperatures: Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial modal properties to 600 °C and 5 Kb. Am. J. Sci. 1981, 281, 1249–1516. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Mayorga, G. Thermodynamics of electrolytes. Ii. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 1973, 77, 2300–2308. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Kim, J.J. Thermodynamics of electrolytes. Iv. Activity and osmotic coefficients for mixed electrolytes. J. Am. Chem. Soc. 1974, 96, 5701–5707. [Google Scholar] [CrossRef]
- Li, D.; Duan, Z. The speciation equilibrium coupling with phase equilibrium in the H2O–CO2–NaCl system from 0 to 250 °C, from 0 to 1000 bar, and from 0 to 5 molality of NaCl. Chem. Geol. 2007, 244, 730–751. [Google Scholar] [CrossRef]
- Tödheide, K.; Franck, E. Das zweiphasengebiet und die kritische kurve im system kohlendioxid–wasser bis zu drucken von 3500 bar. Z. Phys. Chem. 1963, 37, 389–401. [Google Scholar] [CrossRef]
- Takenouchi, S.; Kennedy, G.C. The solubility of carbon dioxide in NaCl solutions at high temperatures and pressures. Am. J. Sci. 1965, 263, 445–454. [Google Scholar] [CrossRef]
- Malinin, S.D.; Savelyeva, N.I. The solubility of CO2 in NaCl and CaCl2 solutions at 25, 50, and 75 °C under elevated CO2 pressures. Geokhimiya 1972, 6, 643–653. [Google Scholar]
- Malinin, S.D.; Kurovskaya, N.A. Investigation of CO2 solubility in a solution of chlorides at elevated temperatures and pressures of CO2. Geokhimiya 1975, 4, 547–551. [Google Scholar]
- Tabasinejad, F.; Moore, R.G.; Mehta, S.A.; Van Fraassen, K.C.; Barzin, Y.; Rushing, J.A.; Newsham, K.E. Water solubility in supercritical methane, nitrogen, and carbon dioxide: Measurement and modeling from 422 to 483 K and pressures from 3.6 to 134 MPa. Ind. Eng. Chem. Res. 2011, 50, 4029–4041. [Google Scholar] [CrossRef]
- Wiebe, R.; Gaddy, V.L.; Heins, C. The solubility of nitrogen in water at 50, 75 and 100 °C from 25 to 1000 atmospheres. J. Am. Chem. Soc. 1933, 55, 947–953. [Google Scholar] [CrossRef]
- Mishnina, T.A.; Avdeeva, O.I.; Bozhovskaya, T.K. Solubility of gases in natural waters depending on temperature, pressure and salt composition. Mater. Vses. Nauchn. Issled. Geol. Inst. 1961, 46, 93–110. [Google Scholar]
- Althaus, K. Messung und Berechnung von Wassergehalten Kohlenwasserstoffhaltiger Gasgemische; Progress Reports VDI; VDI-Verlag: Düsseldorf, Germany, 1999; p. 590. [Google Scholar]
- Namiot, A.Y.; Bondareva, M.M. Solubility of Gases in Water; Gostekhizdat: Moscow, Russia, 1959. [Google Scholar]
- Stenphan, E.L.; Hatfield, N.S.; Peoples, R.S.; Pray, H.A.H. Solubility of Gases in Water and in Aqueous Uranyl Salt Solutions at Elevated Temperatures and Pressures; BMI-1067; Battelle Memorial Inst.: Columbus, OH, USA, 1956. [Google Scholar]
- Pray, H.A.; Stephan, E.F. Solubility of Hydrogen in Uanyl Sulphate Solutions at Elevated Temperatures; BMI-840; Battelle Memorial Inst.: Columbus, OH, USA, 1953. [Google Scholar]
- Yasunishi, A. Solubility of Oxygen in Aqueous Electrolyte Solutions. Kagaku Kogaku Rombunshu 1978, 4, 185–189. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, M.; Yang, G.; Han, B. Solubility of CO2 in aqueous solutions of NaCl, KCl, CaCl2 and their mixed salts at different temperatures and pressures. J. Supercrit. Fluids 2011, 56, 125–129. [Google Scholar] [CrossRef]
- Polya, D.A.; Woolley, E.M.; Simonson, J.M.; Mesmer, R.E. The enthalpy of dilution and thermodynamics of Na2CO3(aq) and NaHCO3(aq) from T = 298 K to T = 523.15 K and pressure of 40 MPa. J. Chem. Thermodyn. 2001, 33, 205–243. [Google Scholar] [CrossRef]
- Pabalan, R.T.; Pitzer, K.S. Thermodynamics of NaOH(aq) in hydrothermal solutions. Geochim. Cosmochim. Acta 1987, 51, 829–837. [Google Scholar] [CrossRef]
- Pitzer, K.S. Thermodynamic properties of aqueous sodium chloride solutions. J. Phys. Chem. Ref. Data 1984, 13, 1–102. [Google Scholar] [CrossRef]
- Ellis, A.J. 750. The effect of pressure on the first dissociation constant of “carbonic acid”. J. Chem. Soc. (Resumed) 1959, 3689–3699. [Google Scholar] [CrossRef]
- Ellis, A.J. The solubility of calcite in sodium chloride solutions at high temperatures. Am. J. Sci. 1963, 261, 259–267. [Google Scholar] [CrossRef]
- Bénézeth, P.; Saldi, G.D.; Dandurand, J.-L.; Schott, J. Experimental determination of the solubility product of magnesite at 50 to 200 °C. Chem. Geol. 2011, 286, 21–31. [Google Scholar] [CrossRef]
- Bénézeth, P.; Berninger, U.-N.; Bovet, N.; Schott, J.; Oelkers, E.H. Experimental determination of the solubility product of dolomite at 50–253 °C. Geochim. Cosmochim. Acta 2018, 224, 262–275. [Google Scholar] [CrossRef]
- Rodrigues, C.F.A.; Dinis, M.A.P.; Lemos de Sousa, M.J. Review of European energy policies regarding the recent “carbon capture, utilization and storage” technologies scenario and the role of coal seams. Environ. Earth Sci. 2015, 74, 2553–2561. [Google Scholar] [CrossRef]












| Tc (K) | Pc (bar) | ||
|---|---|---|---|
| CO2 | 304.2 a | 73.83 a | 0.2236 a |
| N2 | 126.2 b | 34.0 b | 0.0377 b |
| O2 | 154.6 b | 49.8 b | 0.021 b |
| H2O | 647.3 a | 221.2 a | 0.3434 a |
| δij | H2O | CO2 | N2 | O2 |
|---|---|---|---|---|
| H2O | - | 0.1896 a | 0.32547 b | 0.20863 c |
| CO2 | 0.1896 a | - | −0.007 d | 0.1140 d |
| N2 | 0.32547 b | −0.007 d | - | −0.0119 c |
| O2 | 0.20863 c | 0.1140 d | −0.0119c | - |
| A0 | A1 | A2 | A3 | A4 | A5 | a1 | a2 | a3 | a4 | ω | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | −10.52624 | 2.3547 × 10−2 | 3972.8 | 0 | −5.8746 × 105 | −1.9194 × 10−5 | 7.29 | 0.92 | 2.07 | −1.23 | −1.6 |
| N2 | 58.453 | −1.818 × 10−3 | −3199 | −17.909 | 27,460 | 0 | 7 | 0 | 0 | 0 | 0 |
| O2 | 7.5001 | −7.8981 × 10−3 | 0 | 0 | −2.0027 × 105 | 0 | 5.7889 | 6.3536 | 3.2528 | −3.0417 | −0.3943 |
| . | b0 | b1 | b2 | b3 | b4 | b5 |
|---|---|---|---|---|---|---|
| a | 0.085 | |||||
| a | 0.183 | |||||
| a | 0.183 | |||||
| a | 0.075 | |||||
| a | 0.051 | |||||
| b | 0.1402 | −595 | −4.025 | 0.01044 | −2.131 × 10−6 | 49,970 |
| b | 0.2804 | −1190 | −8.05 | 0.02088 | −4.262 × 10−6 | 99,940 |
| b | 0.2804 | −1190 | −8.05 | 0.02088 | −4.262 × 10−6 | 99,940 |
| b | 0.1402 | −595 | −4.025 | 0.01044 | −2.131 × 10−6 | 49,970 |
| b | 0.0371 | |||||
| c | 0.19997 | |||||
| c | 0.31715 | |||||
| c | 0.35135 | |||||
| c | 0.15022 | |||||
| c | 0.14383 | |||||
| b | −0.015 | |||||
| b | −1.16 × 10−2 | |||||
| b | −0.58 × 10−2 | |||||
| b | −0.58 × 10−2 | |||||
| b | −1.16 × 10−2 | |||||
| b | −1.16 × 10−2 | |||||
| b | −2.32 × 10−2 | |||||
| b | −1.16 × 10−2 | |||||
| b | −2.32 × 10−2 |
| Pitzer Parameters | Literature Sources |
|---|---|
| , , , | Duan and Li [20] |
| , | Appelo [30] |
| , , | Christov and Moller [19] |
| , , | Christov and Moller [19] |
| , | Greenberg and Moller [18], Christov and Moller [19] |
| , , | Christov and Moller [19] |
| , , | Christov and Moller [19] |
| , , | Polya et al. [48] |
| , , | Polya et al. [48] |
| , , | Pabalan and Pitzer [49] |
| , , | Pitzer [50] |
| , , , , , | Li and Duan [34] |
| , | Christov and Moller [19] |
| Pabalan and Pitzer [49] | |
| ,, , , , , | Li and Duan [34], Duan and Li [20] |
| , , , | Appelo [30] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ahmed, R.; Li, X. Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure. Energies 2018, 11, 2627. https://doi.org/10.3390/en11102627
Li J, Ahmed R, Li X. Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure. Energies. 2018; 11(10):2627. https://doi.org/10.3390/en11102627
Chicago/Turabian StyleLi, Jun, Raheel Ahmed, and Xiaochun Li. 2018. "Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure" Energies 11, no. 10: 2627. https://doi.org/10.3390/en11102627
APA StyleLi, J., Ahmed, R., & Li, X. (2018). Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure. Energies, 11(10), 2627. https://doi.org/10.3390/en11102627





