Fabrication of PEO-PMMA-LiClO4-Based Solid Polymer Electrolytes Containing Silica Aerogel Particles for All-Solid-State Lithium Batteries
Abstract
:1. Introduction
2. Materials and Methods
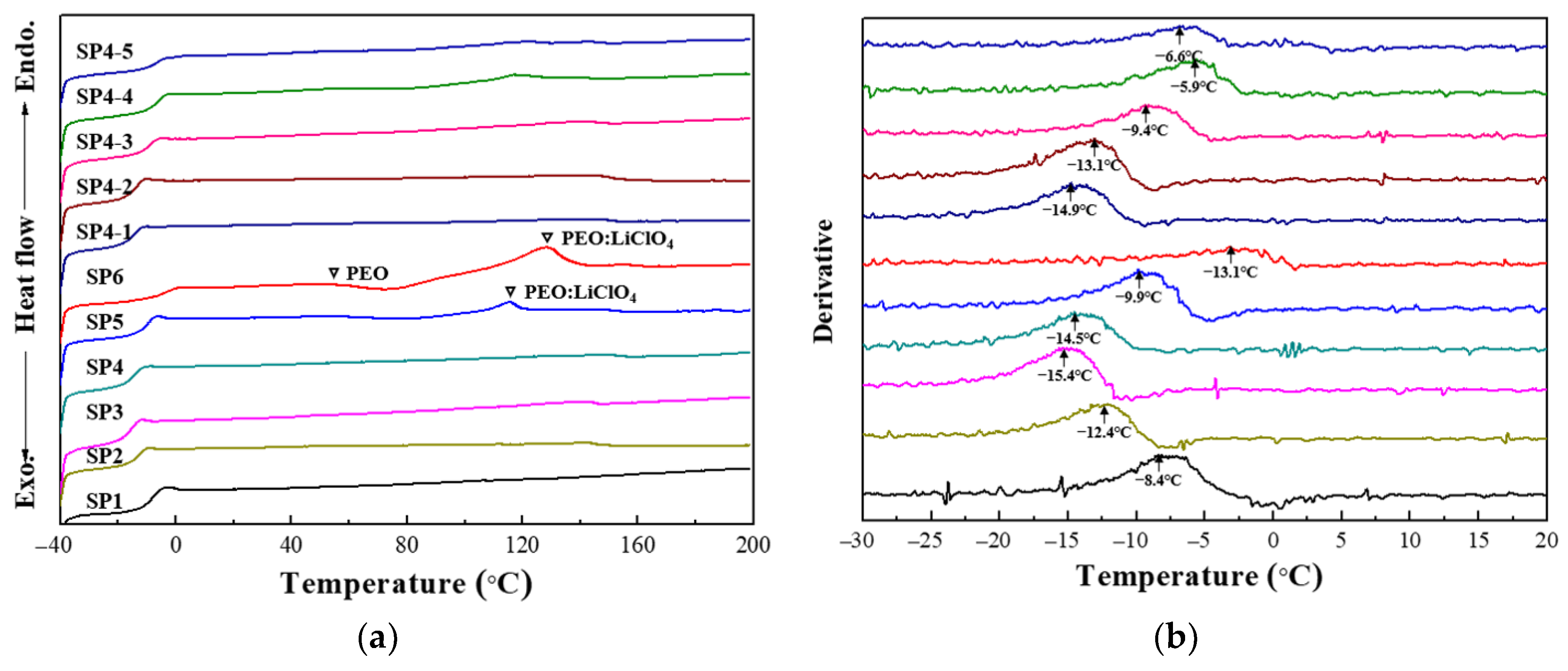
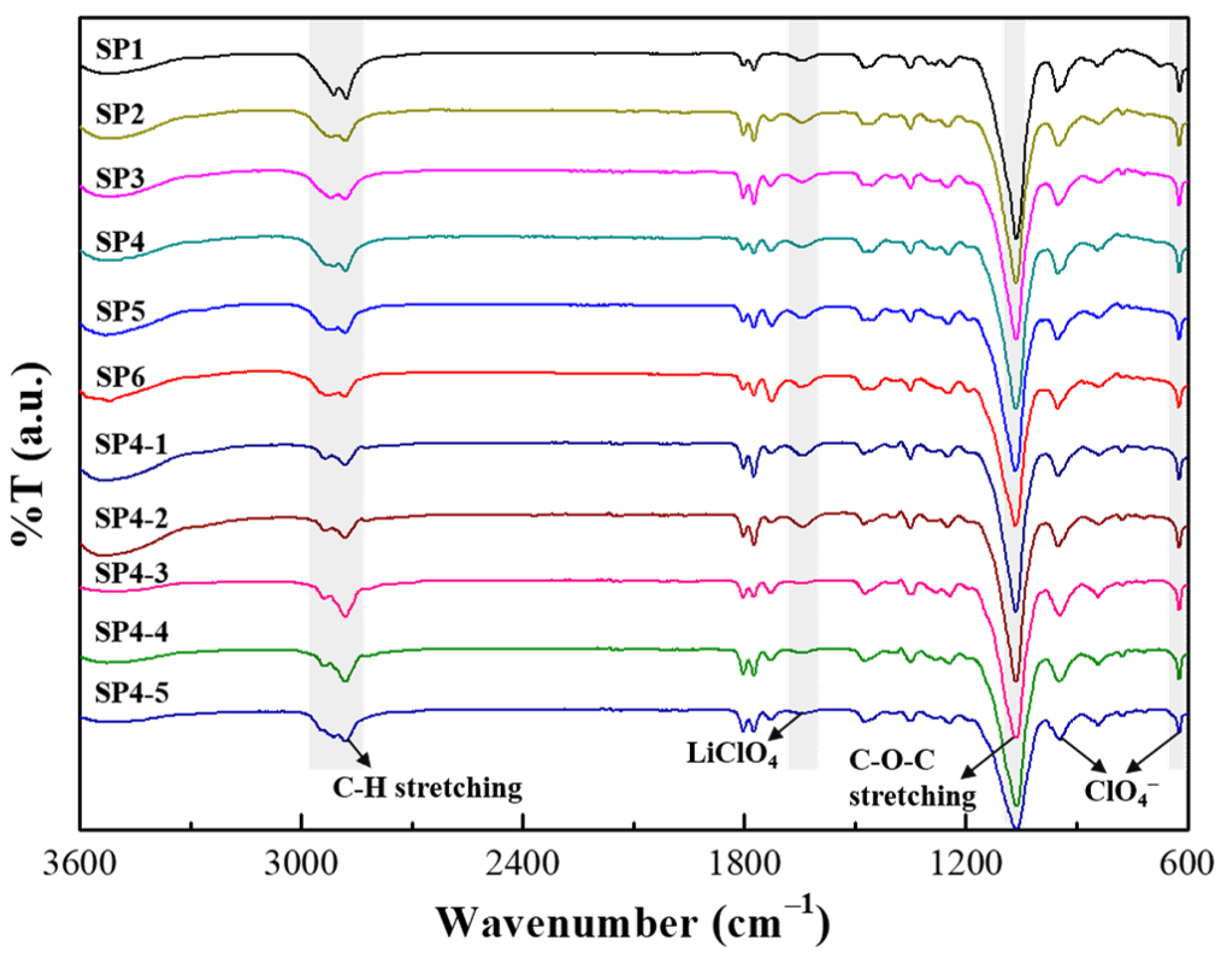
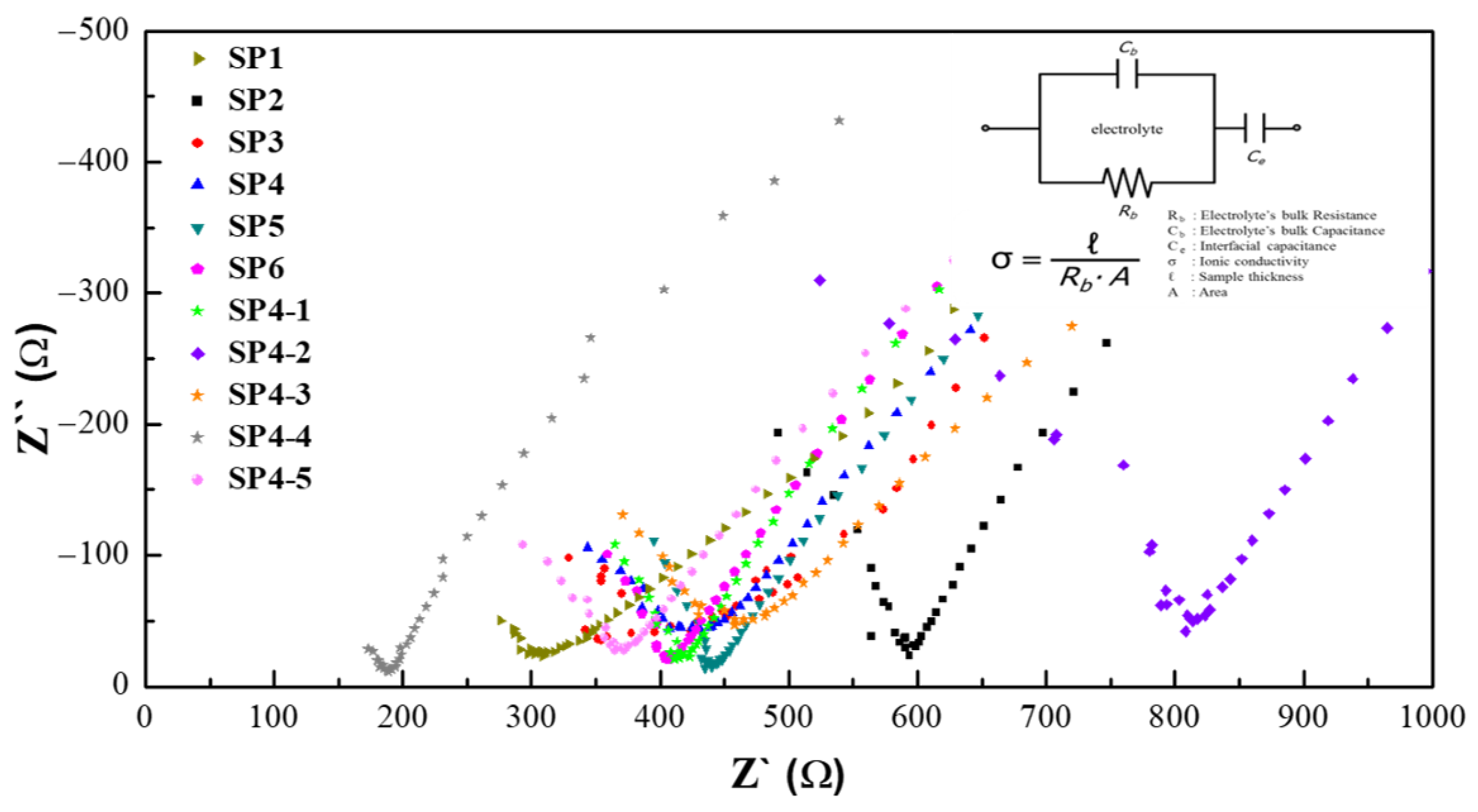
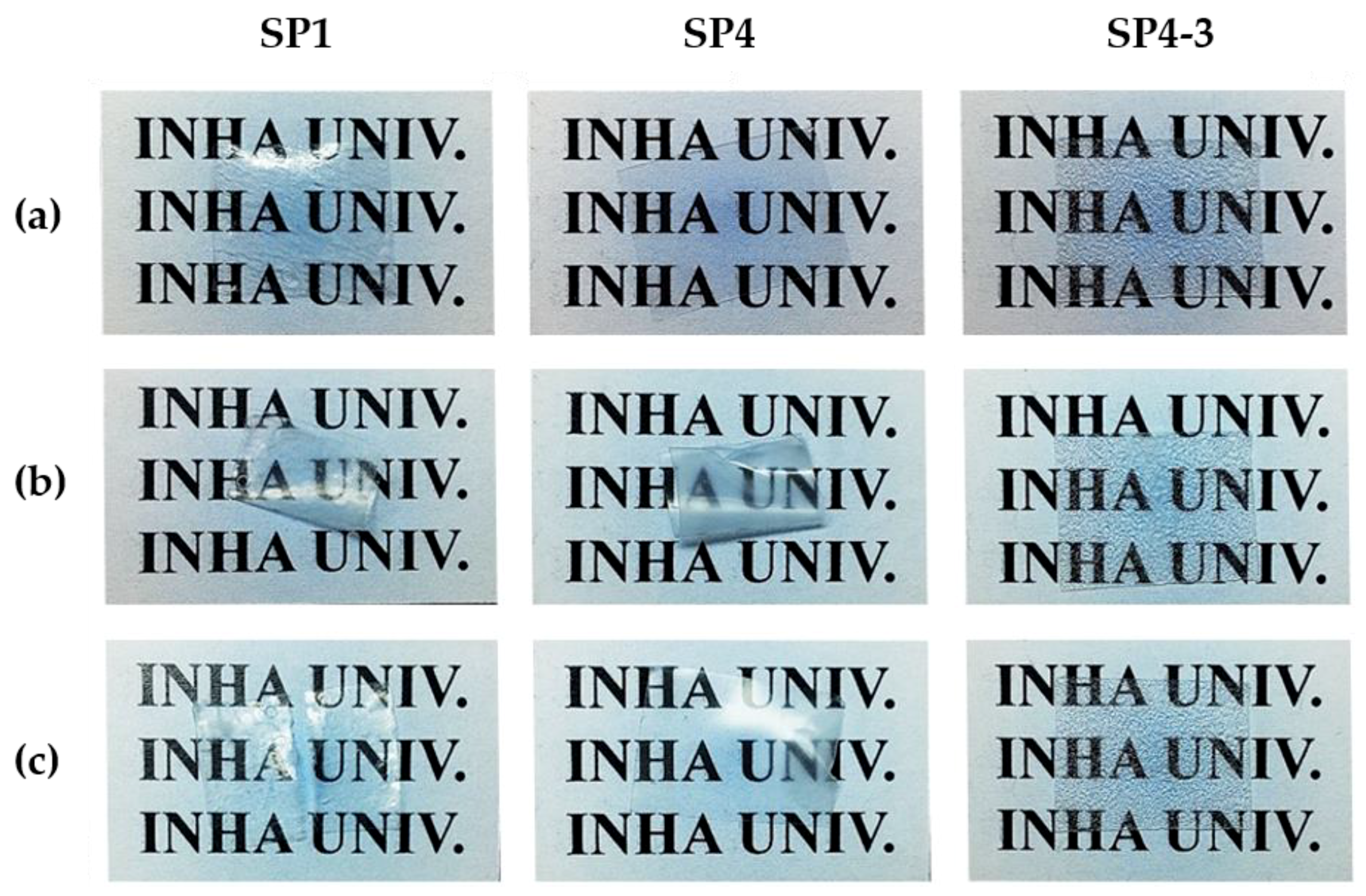
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, R.; Nam, H.O.; Ko, W.I.; Jang, H. National Options for a Sustainable Nuclear Energy System: MCDM Evaluation Using an Improved Integrated Weighting Approach. Energies 2017, 10, 2017. [Google Scholar] [CrossRef]
- Kang, Y.K.; Lee, C.J. Polymer electrolytes for lithium polymer batteries. Polym. Sci. Technol. 2003, 14, 396–406. [Google Scholar]
- Kermani, G.; Sahraei, E. Review: Characterization and Modeling of the Mechanical Properties of Lithium-Ion Batteries. Energies 2017, 10, 1730. [Google Scholar] [CrossRef]
- Park, M.; Jung, W.D.; Choi, S.; Son, K.; Jung, H.; Kim, B.; Lee, H.; Lee, J.; Kim, H. Microscopic analysis of high lithium-ion conducting glass-ceramic sulfides. J. Korean Ceram. Soc. 2016, 53, 568–573. [Google Scholar] [CrossRef]
- Shin, R.H.; Son, S.I.; Lee, S.M.; Han, Y.S.; Kim, Y.D.; Ryu, S.S. Effect of Li3BO3 additive on densification and ion conductivity of garnet-type Li7La3Zr2O12 solid electrolytes of all-solid-state lithium-ion batteries. J. Korean Ceram. Soc. 2016, 53, 712–718. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef] [PubMed]
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Liang, G.; Xu, J.; Xu, W.; Shen, X.; Zhang, H.; Yao, M. Effect of filler-polymer interactions on the crystalline morphology of PEO-based solid polymer electrolytes by Y2O3 nano-fillers. Polym. Compos. 2011, 32, 511–518. [Google Scholar] [CrossRef]
- Jung, Y.C.; Park, M.S.; Doh, C.H.; Kim, D.W. Organic-inorganic hybrid solid electrolytes for solid-state lithium cells operating at room temperature. Electrochim. Acta 2016, 218, 271–277. [Google Scholar] [CrossRef]
- Benedicta, T.J.; Banumathia, S.; Veluchamya, A.; Gangadharana, R.; Ahamadb, A.Z.; Rajendranb, S. Characterization of plasticized solid polymer electrolyte by XRD and AC impedance methods. J. Power Sources 1998, 75, 171–174. [Google Scholar] [CrossRef]
- Michael, M.S.; Jacob, M.M.E.; Prabaharan, S.R.S.; Radhakrishna, S. Enhanced lithium ion transport in PEO-based solid polymer electrolytes employing a novel class of plasticizers. Solid State Ion. 1997, 98, 167–174. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Gopi, S.; Kathiresan, M.; Bose, S.; Gowd, E.B.; Nair, J.R.; Angulakshmi, N.; Meligrana, G.; Bella, F.; Gerbaldi, C.; et al. Metal organic framework laden poly (ethylene oxide) based composite electrolytes for all-solid-state Li-S and Li-metal polymer batteries. Electrochim. Acta 2018, 285, 355–364. [Google Scholar] [CrossRef]
- Xu, G.; Nie, P.; Dou, H.; Ding, B.; Li, L.; Zhang, X. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Hong, S.K.; Hwang, H.J. Fabrication of Li-polymer/silica aerogel nanocomposite electrolyte for an all-solid-state lithium battery. Ceram. Int. 2013, 39, 9659–9663. [Google Scholar] [CrossRef]
- Lee, L.G.; Park, S.J.; Kim, S. Study on ionic conductivity and crystallinity of PEO/PMMA polymer composite electrolytes containing TiO2 filler. Korean Chem. Eng. Res. 2011, 49, 758–763. [Google Scholar] [CrossRef]
- Liang, B.; Tang, S.; Jiang, Q.; Chen, C.; Chen, X.; Li, S.; Yan, X. Preparation and characterization of PEO-PMMA polymer composite electrolytes doped with nano-Al2O3. Electrochim. Acta 2015, 169, 334–341. [Google Scholar] [CrossRef]
- Radzir, N.N.M.; Hanifah, S.A.; Ahmad, A.; Hassan, N.H.; Bella, F. Effect of lithium bis(trifluoromethylsulfonyl)imide salt-doped UV-cured glycidyl methacrylate. J. Solid State Electrochem. 2015, 19, 3079–3085. [Google Scholar] [CrossRef]
- Colò, F.; Bella, F.; Nair, J.R.; Gerbaldi, C. Light-cured polymer electrolytes for safe, low-cost and sustainable sodium-ion batteries. J. Power Sources 2017, 365, 293–302. [Google Scholar] [CrossRef]
- Bella, F.; Colò, F.; Nair, J.R.; Gerbaldi, C. Photopolymer Electrolytes for Sustainable, Upscalable, Safe, and Ambient-Temperature Sodium-Ion Secondary Batteries. ChemSusChem 2015, 8, 3668–3676. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Sivakumar, M.; Subadevi, R. Investigations on the effect of various plasticizers in PVA–PMMA solid polymer blend electrolytes. Mater. Lett. 2004, 58, 641–649. [Google Scholar] [CrossRef]
- Dionísio, M.; Fernandes, A.C.; Mano, J.F.; Correia, N.T.; Sousa, R.C. Relaxation studies in PEO/PMMA blends. Macromolecules 2000, 33, 1002–1011. [Google Scholar] [CrossRef]
- Flora, X.H.; Ulaganathan, M.; Rajendran, S. Influence of lithium salt concentration on PAN-PMMA blend polymer electrolytes. Int. J. Electrochem. Sci. 2012, 7, 7451–7462. [Google Scholar]
- Meyer, W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Fullerton-Shirey, S.K.; Maranas, J.K. Effect of LiClO4 on the structure and mobility of PEO-based solid polymer electrolytes. Macromoleculecules. 2009, 42, 2142–2156. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, Y.; Wang, L.; Pang, M.J.; Chen, L. Characterization of (PEO)LiClO4-Li1.3Al0.3Ti1.7(PO4)3 composite polymer electrolytes with different molecular weights of PEO. J. Appl. Polym. Sci. 2006, 102, 4269–4275. [Google Scholar] [CrossRef]
- Robitaille, C.D.; Fauteux, D. Phase diagrams and conductivity characterization of some PEO-LiX electrolytes. J. Electrochem. Soc. 1986, 133, 315–325. [Google Scholar] [CrossRef]
- Fullerton-Shirey, S.K.; Maranas, J.K. Structure and mobility of PEO/LiClO4 solid polymer electrolytes filled with Al2O3 nanoparticles. J. Phys. Chem. C 2010, 114, 9196–9206. [Google Scholar] [CrossRef]
- Kumar, M.; Chung, J.S.; Hur, S.H. Controlled atom transfer radical polymerization of MMA onto the surface of high-density functionalized graphene oxide. Nanoscale Res. Lett 2014, 9, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Munichandraiah, N.; Scanlon, L.G.; Marsh, R.A.; Kumar, B.; Sircar, A.K. Influence of zeolite on electrochemical and physicochemical properties of polyethylene oxide solid electrolyte. J. Appl. Electrochem. 1995, 25, 857–863. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, W.; Choi, B.K. Relation between glass transition and melting of PEO–salt complexes. Electrochim. Acta 2000, 45, 1473–1477. [Google Scholar] [CrossRef]
- Kweon, J.-O.; Noh, S.-T. Thermal, thermomechanical, and electrochemical characterization of the organic–inorganic hybrids poly(ethylene oxide) (PEO)–silica and PEO–silica–LiClO4. J. Appl. Polym. Sci. 2001, 81, 2471–2479. [Google Scholar] [CrossRef]
- Licoccia, S.; Trombetta, M.; Capitani, D.; Proietti, N.; Romagnoli, P.; Vona, M.L.D. ATR–FTIR and NMR spectroscopic studies on the structure of polymeric gel electrolytes for biomedical applications. Polymer 2005, 46, 4670–4675. [Google Scholar] [CrossRef]
- Shukla, N.; Thakur, A.K. Role of salt concentration on conductivity optimization and structural phase separation in a solid polymer electrolyte based on PMMA-LiClO4. Ionics 2009, 15, 357–367. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Hu, H.; Yuan, W.; Lu, L.; Zhao, H.; Jia, Z.; Baker, G.L. Low glass transition temperature polymer electrolyte prepared from ionic liquid grafted polyethylene oxide. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2104–2110. [Google Scholar] [CrossRef]
- Wieczorek, W.; Florjanczyk, Z.; Stevens, J.R. Composite polyether based solid electrolytes. Electrochim. Acta 1995, 40, 2251–2258. [Google Scholar] [CrossRef]
- Croce, F.; Persi, L.; Scrosati, B.; Serraino-Fiory, F.; Plichta, E.; Hendrickson, M.A. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim. Acta 2011, 46, 2457–2461. [Google Scholar] [CrossRef]
- Jayathilaka, P.A.R.D.; Dissanayake, M.A.K.L.; Albinssona, I.; Mellander, B.-E. Effect of nano-porous Al2O3 on thermal, dielectric and transport properties of the (PEO)9LiTFSI polymer electrolyte system. Electrochim. Acta 2002, 47, 3257–3268. [Google Scholar] [CrossRef]
- Panero, S.; Scrosati, B.; Sumathipala, H.H.; Wieczorek, W. Dual-composite polymer electrolytes with enhanced transport properties. J. Power Sources 2007, 167, 510–514. [Google Scholar] [CrossRef]
- Vignarooban, K.; Dissanayake, M.A.K.L.; Albinsson, I.; Mellander, B.-E. Effect of TiO2 nano-filler and EC plasticizer on electrical and thermal properties of poly (ethylene oxide) (PEO) based solid polymer electrolytes. Solid State Ion. 2014, 266, 25–28. [Google Scholar] [CrossRef]
- Parale, V.G.; Lee, K.-Y.; Park, H.-H. Flexible and transparent silica aerogels: An overview. J. Korean Ceram. Soc. 2017, 54, 184–199. [Google Scholar] [CrossRef]


| Sample Name | PEO:PMMA (Molar Ratio) | Silica Aerogel (wt%) |
|---|---|---|
| SP1 | 1:0 | - |
| SP2 | 12:1 | - |
| SP3 | 10:1 | - |
| SP4 | 8:1 | - |
| SP5 | 4:1 | - |
| SP6 | 2:1 | - |
| SP4-1 | 8:1 | 1 |
| SP4-2 | 8:1 | 2 |
| SP4-3 | 8:1 | 4 |
| SP4-4 | 8:1 | 8 |
| SP4-5 | 8:1 | 16 |
| Sample | PEO:PMMA (Molar Ratio) | Silica Aerogel (wt%) | Ion Conductivity (S·cm−1, at 30 °C) |
|---|---|---|---|
| SP1 | 1:0 | - | 2.36 × 10−5 |
| SP2 | 12:1 | - | 3.64 × 10−5 |
| SP3 | 10:1 | - | 6.18 × 10−5 |
| SP4 | 8:1 | - | 6.70 × 10−5 |
| SP5 | 4:1 | - | 4.63 × 10−5 |
| SP6 | 2:1 | - | 4.40 × 10−5 |
| SP4-1 | 8:1 | 1 | 2.52 × 10−5 |
| SP4-2 | 8:1 | 2 | 2.83 × 10−5 |
| SP4-3 | 8:1 | 4 | 5.37 × 10−5 |
| SP4-4 | 8:1 | 8 | 1.35 × 10−4 |
| SP4-5 | 8:1 | 16 | 1.02 × 10−4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.S.; Jung, H.-A.; Hwang, H. Fabrication of PEO-PMMA-LiClO4-Based Solid Polymer Electrolytes Containing Silica Aerogel Particles for All-Solid-State Lithium Batteries. Energies 2018, 11, 2559. https://doi.org/10.3390/en11102559
Lim YS, Jung H-A, Hwang H. Fabrication of PEO-PMMA-LiClO4-Based Solid Polymer Electrolytes Containing Silica Aerogel Particles for All-Solid-State Lithium Batteries. Energies. 2018; 11(10):2559. https://doi.org/10.3390/en11102559
Chicago/Turabian StyleLim, Ye Sol, Hyun-Ah Jung, and Haejin Hwang. 2018. "Fabrication of PEO-PMMA-LiClO4-Based Solid Polymer Electrolytes Containing Silica Aerogel Particles for All-Solid-State Lithium Batteries" Energies 11, no. 10: 2559. https://doi.org/10.3390/en11102559




