Li-B Alloy as an Anode Material for Stable and Long Life Lithium Metal Batteries
Abstract
:1. Introduction
2. Experimental
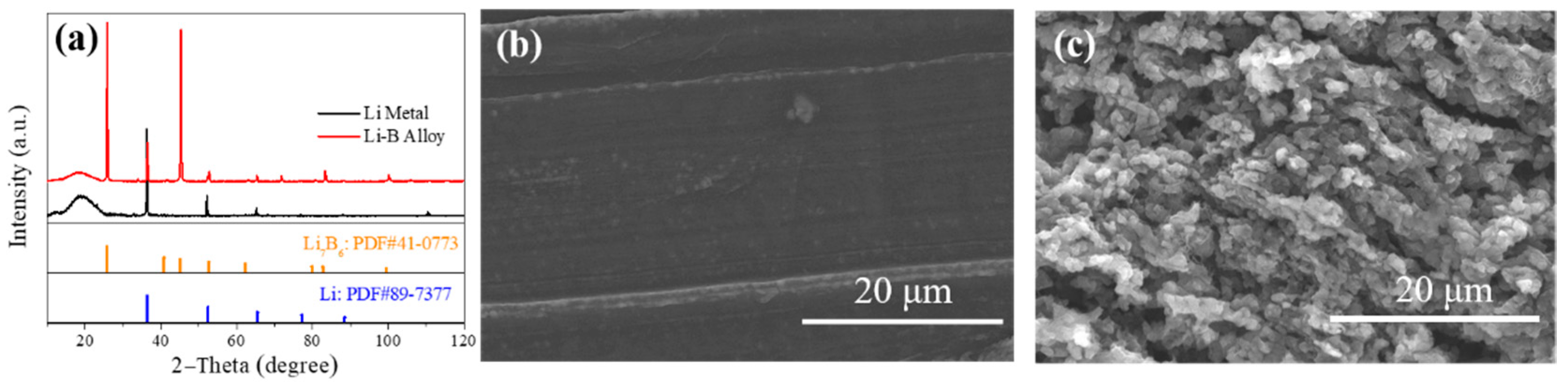
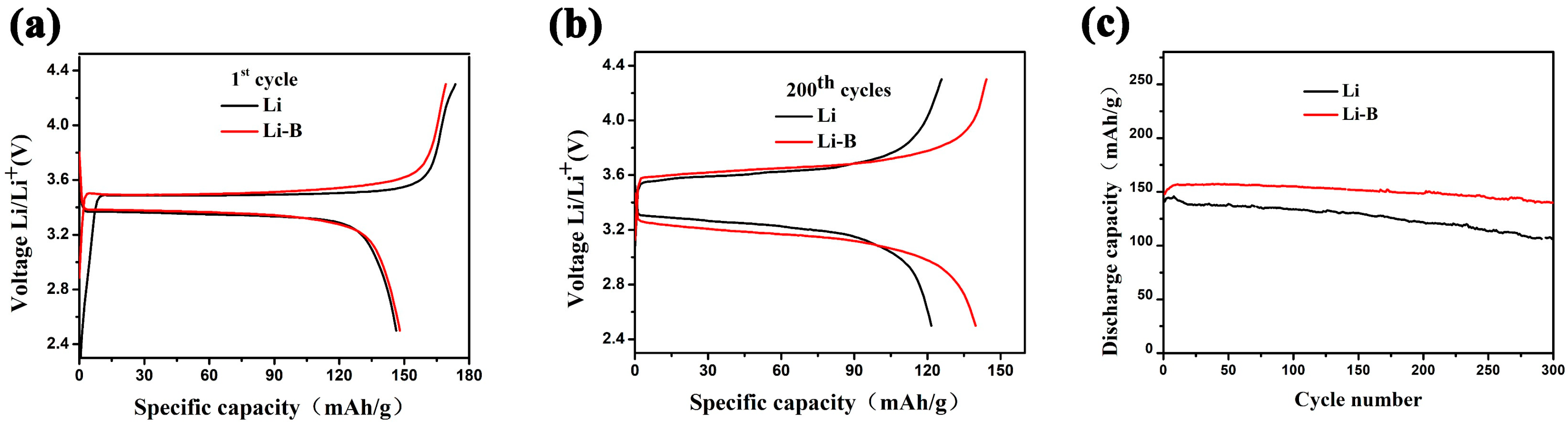
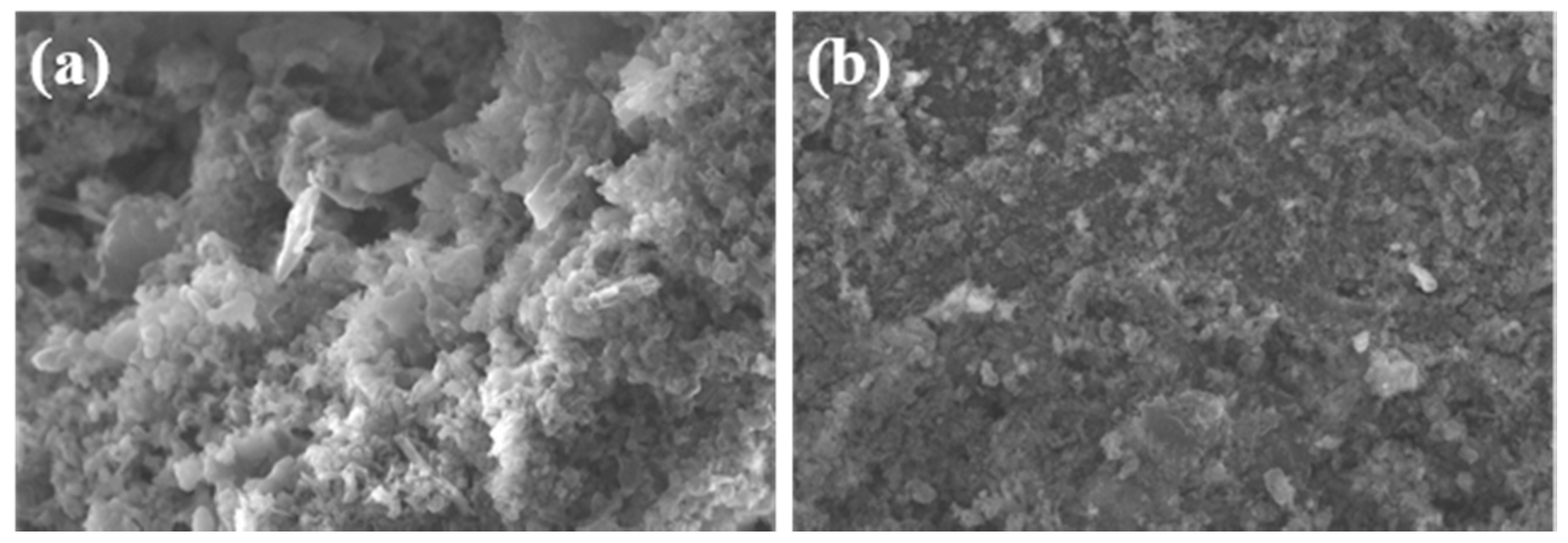
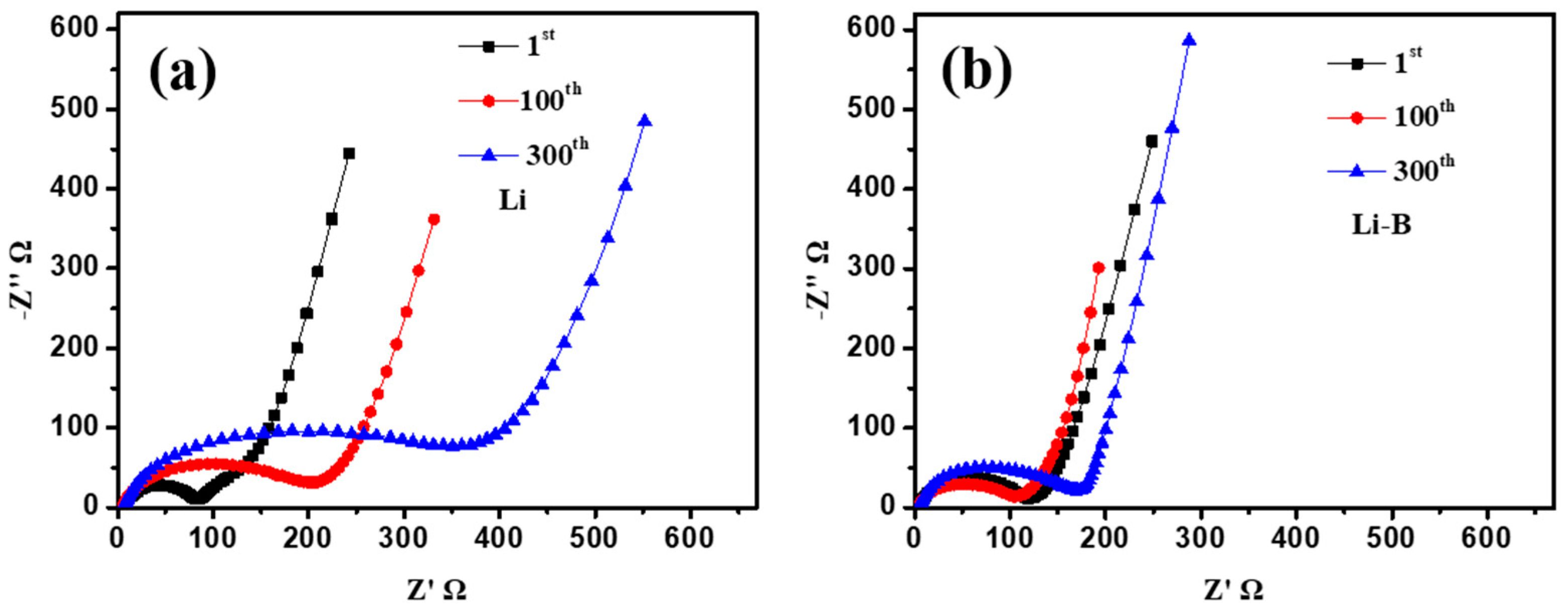
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B. Evolution of Strategies for Modern Rechargeable Batteries. Acc. Chem. Res. 2013, 46, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Zu, C.X.; Li, H. Thermodynamic analysis on energy densities of batteries. Energy Environ. Sci. 2011, 4, 2614–2624. [Google Scholar] [CrossRef]
- Hesse, H.C.; Schimpe, M.; Kucevic, D.; Jossen, A. Lithium-ion battery storage for the grid—A review of stationary battery storage system design tailored for applications in modern power grids. Energies 2017, 10, 2107. [Google Scholar] [CrossRef]
- Panchal, S.; Mcgrory, J.; Kong, J.; Fraser, R.; Fowler, M.; Dincer, I.; Agelin-Chaab, M. Cycling degradation testing and analysis of a LiFePO4 battery at actual conditions. Int. J. Energy Res. 2017, 41, 2565–2575. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Cheng, X.B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene Carbonate Additives to Render Uniform Li Deposits in Lithium Metal Batteries. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale Nucleation and Growth of Electrodeposited Lithium Metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Fan, C.; Wang, L.; Wang, Q.; Zhao, M.; Zhou, A.; Li, J. Extremely Accessible Potassium Nitrate (KNO3) as the Highly Efficient Electrolyte Additive in Lithium Battery. ACS Appl. Mater. Interfaces 2016, 8, 15399–15405. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Liang, X.; Shyamsunder, A.; Nazar, L.F. An In Vivo Formed Solid Electrolyte Surface Layer Enables Stable Plating of Li Metal. Joule 2017, 1, 871–886. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, F.; Bai, Y.; Li, Y.; Chen, G.; Wang, Z.; Wu, C. Regulating Li deposition by constructing LiF-rich host for dendrite-free lithium metal anode. Energy Storage Mater. 2019, 16, 411–418. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Qian, J.; Adams, B.D.; Zheng, J.; Xu, W.; Henderson, W.A.; Wang, J.; Bowden, M.E.; Xu, S.; Hu, J.; Zhang, J.G. Anode-Free Rechargeable Lithium Metal Batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, W.; Zhuo, D.; Zheng, G.; Lu, Z.; Liu, K.; Cui, Y. Core–Shell Nanoparticle Coating as an Interfacial Layer for Dendrite-Free Lithium Metal Anodes. ACS Cent. Sci. 2017, 3, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Wang, Q.; Li, W.; Wu, B.; Jia, W.; Wang, Y.; Li, J.; Li, H. Long lifespan lithium metal anodes enabled by Al2O3 sputter coating. Energy Storage Mater. 2018, 10, 16–23. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Jia, W.; Chen, S.; Gao, P.; Li, J. Li metal coated with amorphous Li3PO4 via magnetron sputtering for stable and long-cycle life lithium metal batteries. J. Power Sources 2017, 342, 175–182. [Google Scholar] [CrossRef]
- Li, N.W.; Yin, Y.X.; Yang, C.P.; Guo, Y.G. An Artificial Solid Electrolyte Interphase Layer for Stable Lithium Metal Anodes. Adv. Mater. 2016, 28, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.; Belin, C.; Crepy, G.; De Guibert, A. Preparation and characterization of lithium-boron alloys: Electrochemical studies as anodes in molten salt media, and comparison with pure lithium-involving systems. J. Mater. Sci. 1992, 27, 240–246. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Z.; Lv, X.; Zhou, G.; Yin, J. Electrochemical studies of LiB compound as anode material for lithium-ion battery. Electrochim. Acta 2006, 51, 5731–5737. [Google Scholar] [CrossRef]
- Duan, B.; Wang, W.; Zhao, H.; Wang, A.; Wang, M.; Yuan, K.; Yu, Z.; Yang, Y. Li-B Alloy as Anode Material for Lithium/Sulfur Battery. ECS Electrochem. Lett. 2013, 2, A47–A51. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Wang, A.; Huang, Y.; Yuan, K.; Yu, Z.; Qiu, J.; Yang, Y. Improved cycle stability and high security of Li-B alloy anode for lithium/sulfur battery. J. Mater. Chem. A 2014, 2, 11660–11665. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Zhou, S.; Tang, C.; Zhai, Q.; Zhang, X.; Wang, R. Li-B Alloy as an Anode Material for Stable and Long Life Lithium Metal Batteries. Energies 2018, 11, 2512. https://doi.org/10.3390/en11102512
Liu Q, Zhou S, Tang C, Zhai Q, Zhang X, Wang R. Li-B Alloy as an Anode Material for Stable and Long Life Lithium Metal Batteries. Energies. 2018; 11(10):2512. https://doi.org/10.3390/en11102512
Chicago/Turabian StyleLiu, Qiang, Sisi Zhou, Cong Tang, Qiaoling Zhai, Xianggong Zhang, and Rui Wang. 2018. "Li-B Alloy as an Anode Material for Stable and Long Life Lithium Metal Batteries" Energies 11, no. 10: 2512. https://doi.org/10.3390/en11102512
APA StyleLiu, Q., Zhou, S., Tang, C., Zhai, Q., Zhang, X., & Wang, R. (2018). Li-B Alloy as an Anode Material for Stable and Long Life Lithium Metal Batteries. Energies, 11(10), 2512. https://doi.org/10.3390/en11102512




