1. Introduction
The employment of absorption systems are a sustainable way to be used in air conditioning applications and reduce the electricity consumption in warm and hot climates. However, they are still more expensive and bulkier than vapor compression systems due to the low coefficient of performance caused basically by the thermodynamic properties of the working fluids and the low heat transfer rate in the components.
The design of the heat exchangers is still complicated in the absorption systems, mainly the absorber component. However, there are passive techniques available to increase the heat and mass transfer such as corrugation, microchannels, and finned surfaces, amongst others. Gutulothu et al. [
1] presented a review of experimental and theoretical studies related to heat exchangers using passive techniques such as tape inserts or nanoparticles. The study describes the results of the heat transfer enhancement at different Reynolds number, Nusselt number, the concentration of nanofluids, and size of the nanoparticle.
Among studies applying passive techniques in the absorbers component of absorption cooling systems, it was found that Lee et al. [
2] analyzed an absorber using a flat plate heat exchanger with NH
3-H
2O. They concluded that the increase of solution flow rate affected slightly the mass transfer process, but improved the heat transfer process. On the other hand, the heat transfer was improved when the vapor flow rate was increased. The authors proposed an experimental correlation for the dimensionless Nusselt and Sherwood numbers.
Cerezo et al. [
3] carried out a theoretical and experimental comparison of an absorber with NH
3-H
2O using a corrugated plate heat exchanger. The absorber heat load and absorption flux obtained the maximum difference of 11.1 and 28.8% between simulation and experimental data.
R. Cardenas and V. Narayanan [
4] studied the NH
3-H
2O absorption process into a constrained microscale film. The solution flows in a microchannel and the ammonia vapor bubbles were injected in cross flow from a porous wall. The result showed that a lower inlet cooling water temperature affects the absorption rate by increasing the local concentration difference between the saturation and bulk values.
Oronel et al. [
5] studied the absorption performance of a plate heat exchanger with NH
3-H
2O and NH
3-(LiNO
3 + H
2O) mixtures at operating conditions of air-cooled absorption system. The results showed that the mass absorption flux and solution heat transfer coefficient was around 1.3 to 1.6 and 1.4 times higher with ternary than the binary mixture. It was by the lower viscosity achieved with the ternary mixture and the high affinity between ammonia and water.
Amaris et al. [
6] compared a tubular bubble absorber with smooth tube and an internally micro-finned tube using NH
3-LiNO
3 at typical operating conditions for absorption chillers. The results showed that with the use of a microchannel tube an absorption rate 1.7 times higher than a smooth tube was obtained at a flow rate of 40 kg/h, also the absorption mass flow rate increases when tube diameter is reduced and decreases when tube length is increased. Moreover, Amaris et al. [
7] analyzed the absorption performance using carbon nanotubes, an internal helical micro-fins and the combination of carbon nanotube and helical micro fins simultaneously in a tubular absorber with NH
3-LiNO
3. The results obtained a maximum absorption mass flux of 1.64 and 1.48 higher with nanoparticles than smooth tubes at 40 and 35 °C, respectively. The simultaneous effect of nanotubes and advanced surface obtained an important improvement in the absorption mass flux at low solution mass flow values.
Triché et al. [
8] studied the NH
3-H
2O heat and mass transfer process in a falling film absorber with a plate heat exchanger. They obtained a maximal relative error of 15% between numerical and experimental data. Besides they concluded that the mass transfer was controlled by the falling film mass transfer resistance while the liquid heat transfer resistance is negligible.
Membrane contactors have been studied as absorber because the use of polymeric hydrophobic microporous membrane contactors can reduce the manufacturing cost. F. Asfand and M. Bourouis [
9] presented a review of this kind of application in absorption systems.
The static mixers have been considered appropriate for the gas-liquid absorption process for a long time now [
10]. However, in its understanding, there is a gap corresponding to the studies related to the ammonia-water absorption along static mixers. This is confirmed in a recent static mixers review, in which this application is absent [
11]. So, recent air-water and advanced studies in an upward co-current gas-liquid flow along a helical static mixer consider the visualization and quantitative of the dispersive mixing of air in a water flow, as well as its simulations in Computational Fluid Dynamic (CFD) software [
12,
13]. These studies give details of the physical processes involved in this mixing process and show the path to be followed by the processes carried out in ammonia-water absorption, where there is a chemical reaction.
The design of the absorber component usually is a complex design due to the heat generated by the absorption of vapor into the liquid phase which should imply high values of heat and mass transfer coefficients. The double pipe heat exchangers are relatively simple to build and with a very low pressure drop, however the poor heat transfer capacity is a disadvantage [
14].
The static mixer has been used in many applications in order to increase the heat transfer; however, there are no studies on absorption process in absorption cooling systems. This paper carries out an experimental and theoretical study of NH3-H2O absorption process at typical operation at air conditioning conditions using a simple double pipe heat exchanger with helical screw static mixers in both cooling water and solution flow rate to increase the heat and mass transfer.
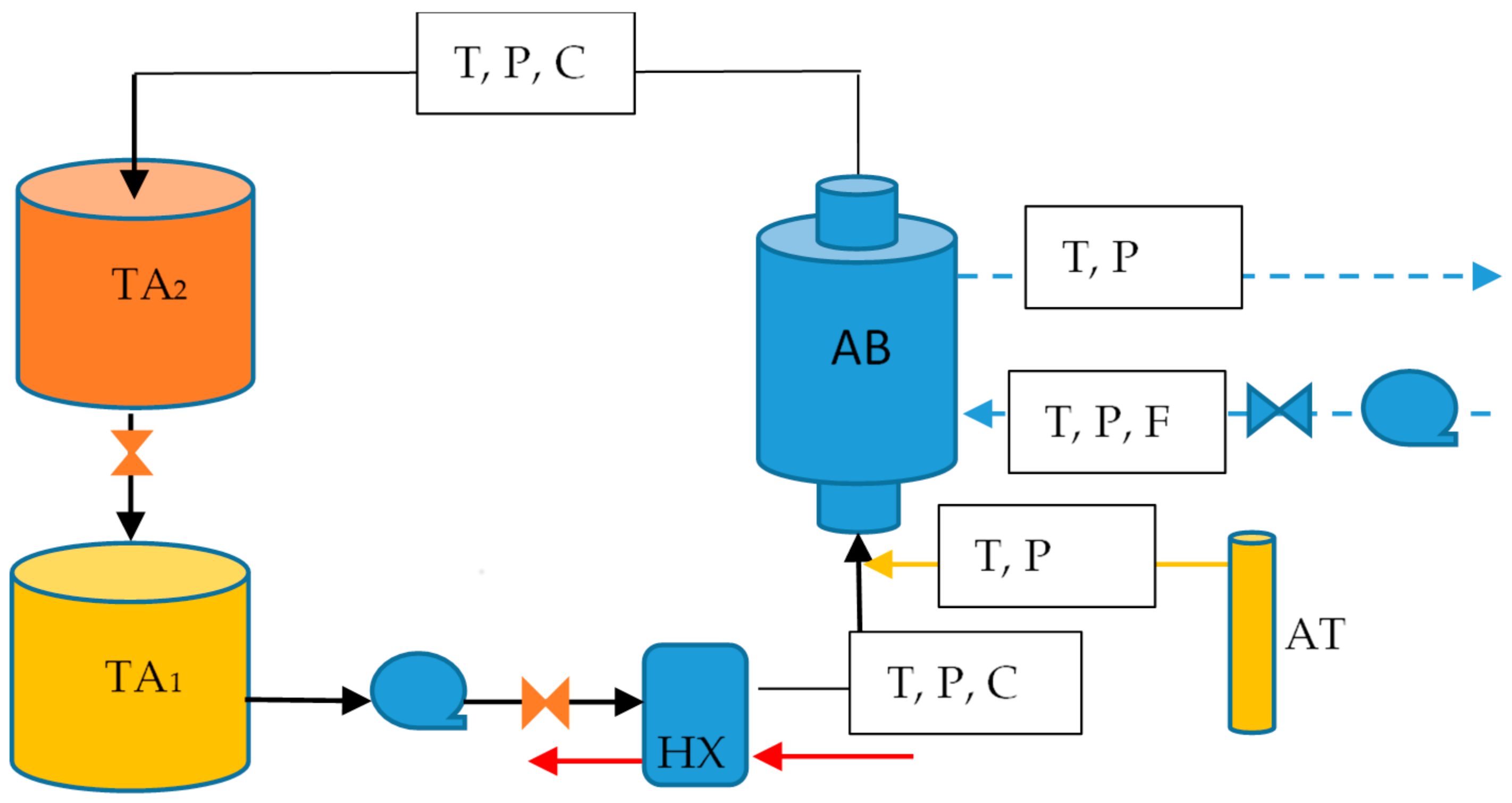
4. Theoretical and Experimental Data Comparison
This section shows the simulation of the bubble absorber using solution heat transfer coefficients obtained from experimental data referred from
Table 3 and
Table 4.
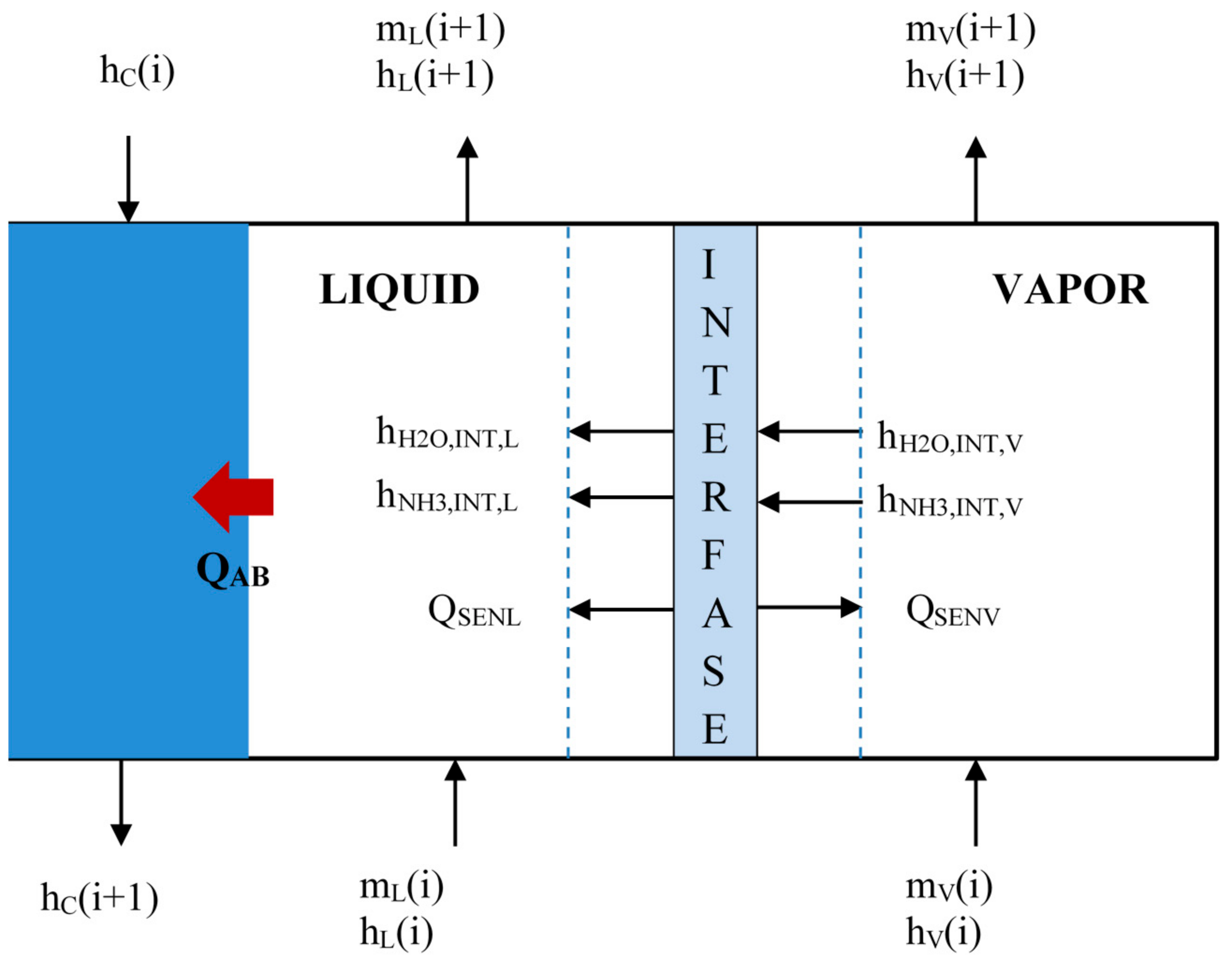
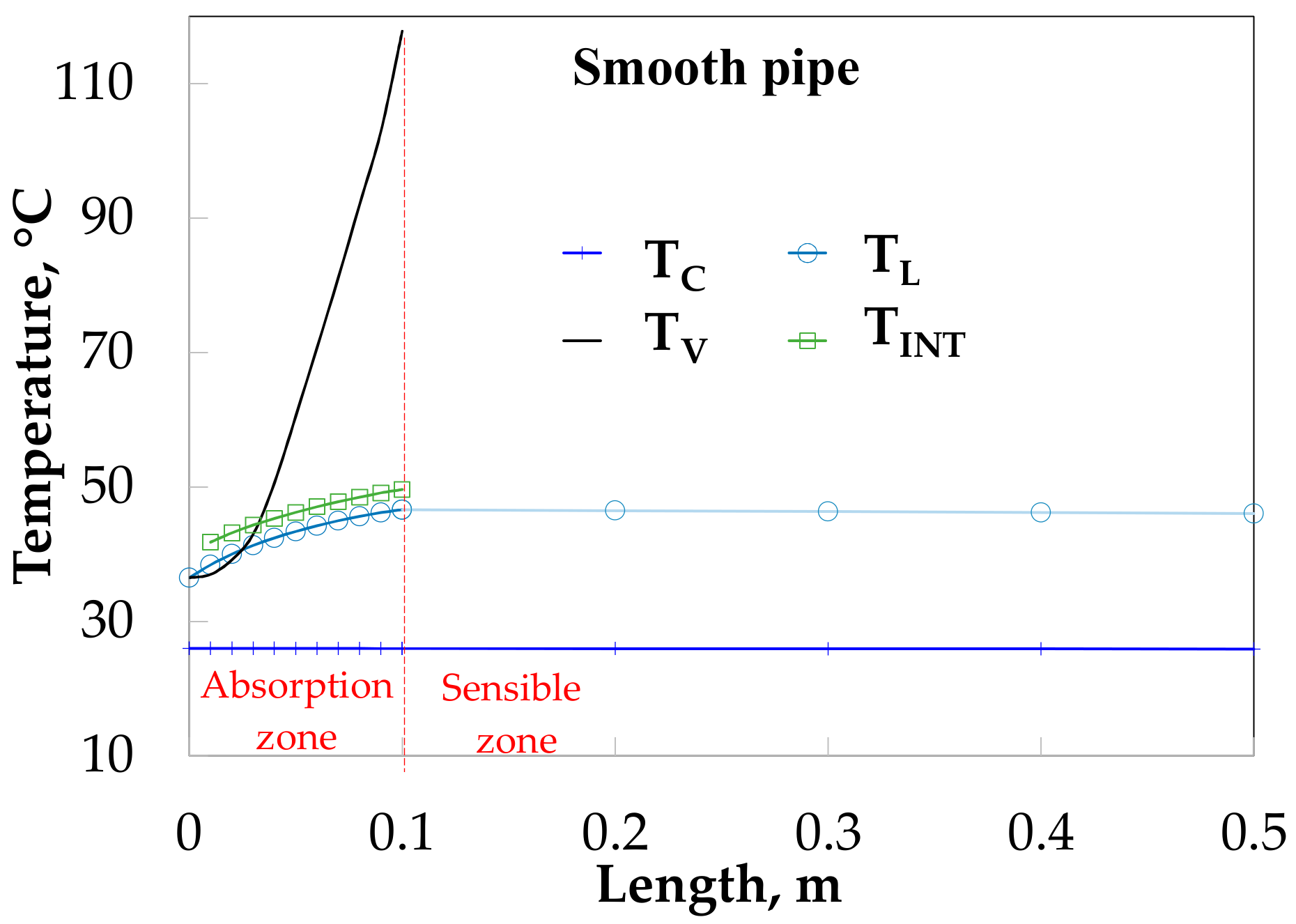
Figure 11 shows that the vapor increases from 36 to 110 °C for SP. The absorption process is considered completed at 0.11 m of absorber length. The solution and interfacial temperatures have a similar tendency in the absorption heat zone and both increase from 36.9 to 44.0 °C and 41.7 to 48.3 °C in the absorption zone, respectively, after that, the solution temperature decrease until 40.8 °C. The cooling water temperature increased from 26.5 to 27.2 °C.
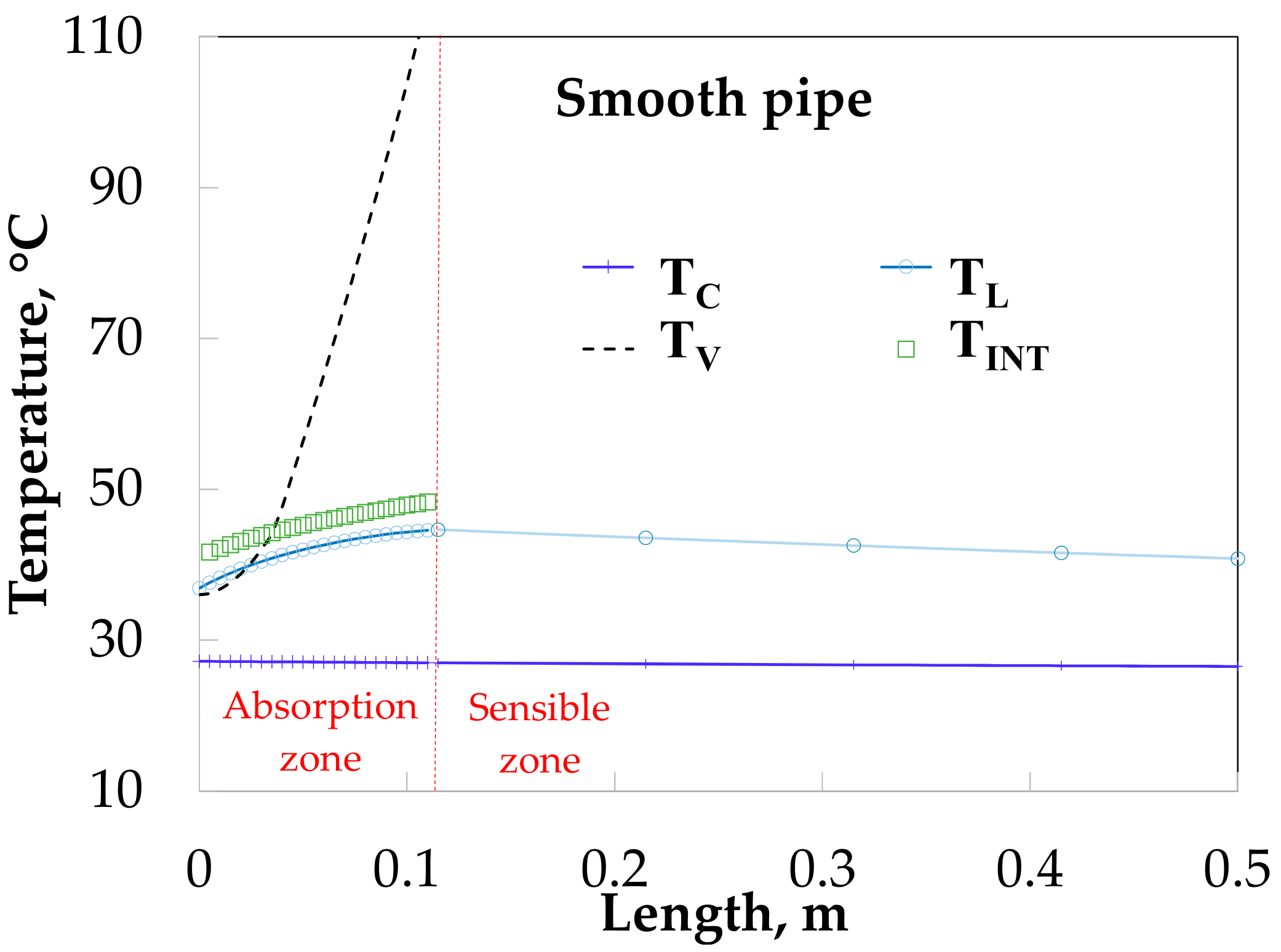
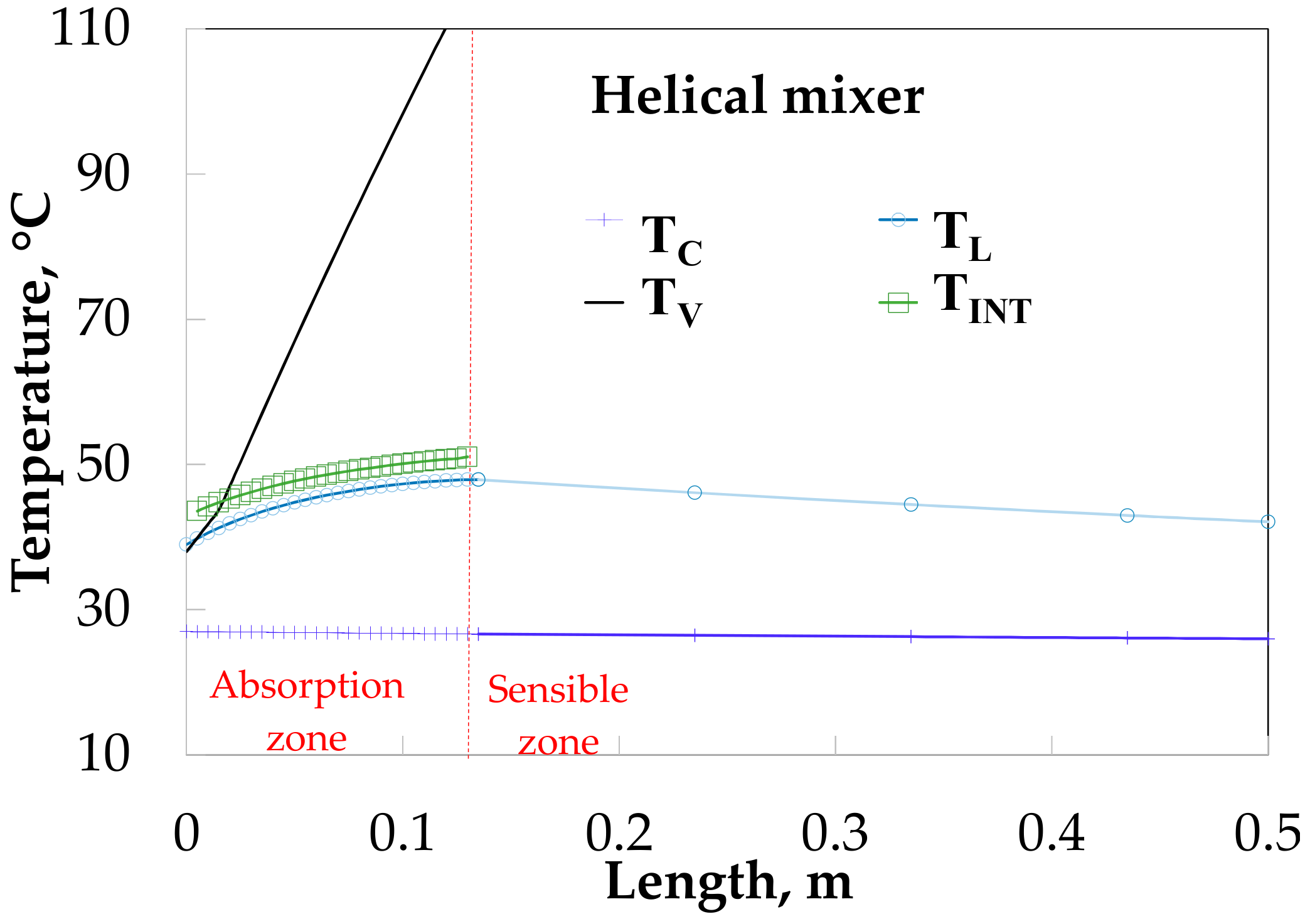
Figure 12 shows the temperature profiles along the absorber with HM. The vapor and solution temperatures have similar behavior for the SP (
Figure 11). The solution temperature increases from 38.0 to 47.9 °C during the absorption process and later decreases to 42.1 °C.
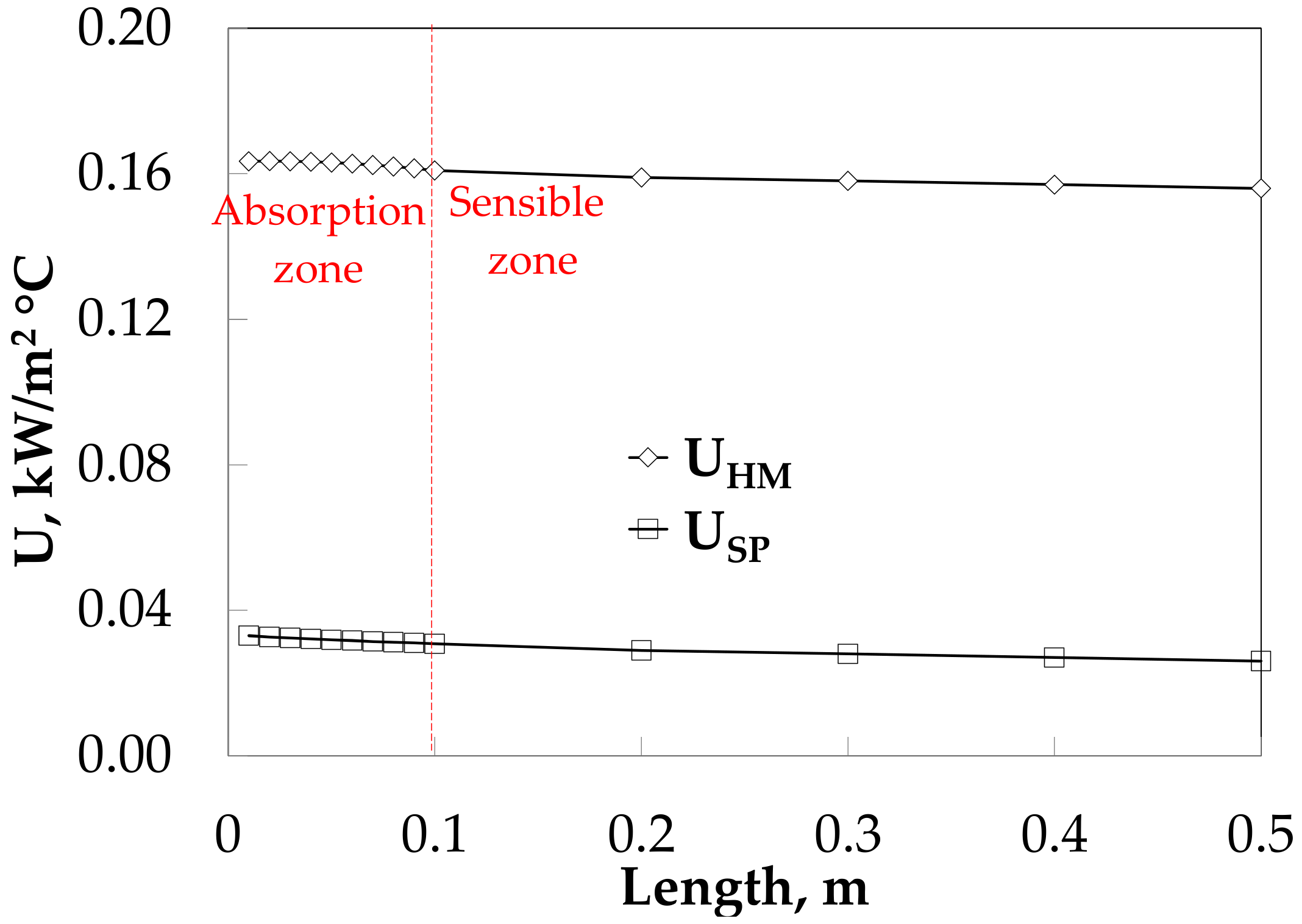
The absorption heat load obtained values of 0.22 and 0.40 kW for SP and HM in the absorption heat zone, and both increase up to 0.72 and 1.14 kW in sensible heat zone respectively, as it can see in
Figure 13.
Table 5 shows a comparison between theoretical and experimental data results. Simulation results were lower than experimental data in general. The difference of heat load between numerical results was 28.0 and 21.9% lower than experimental data for SP and HM, respectively.
5. Discussion
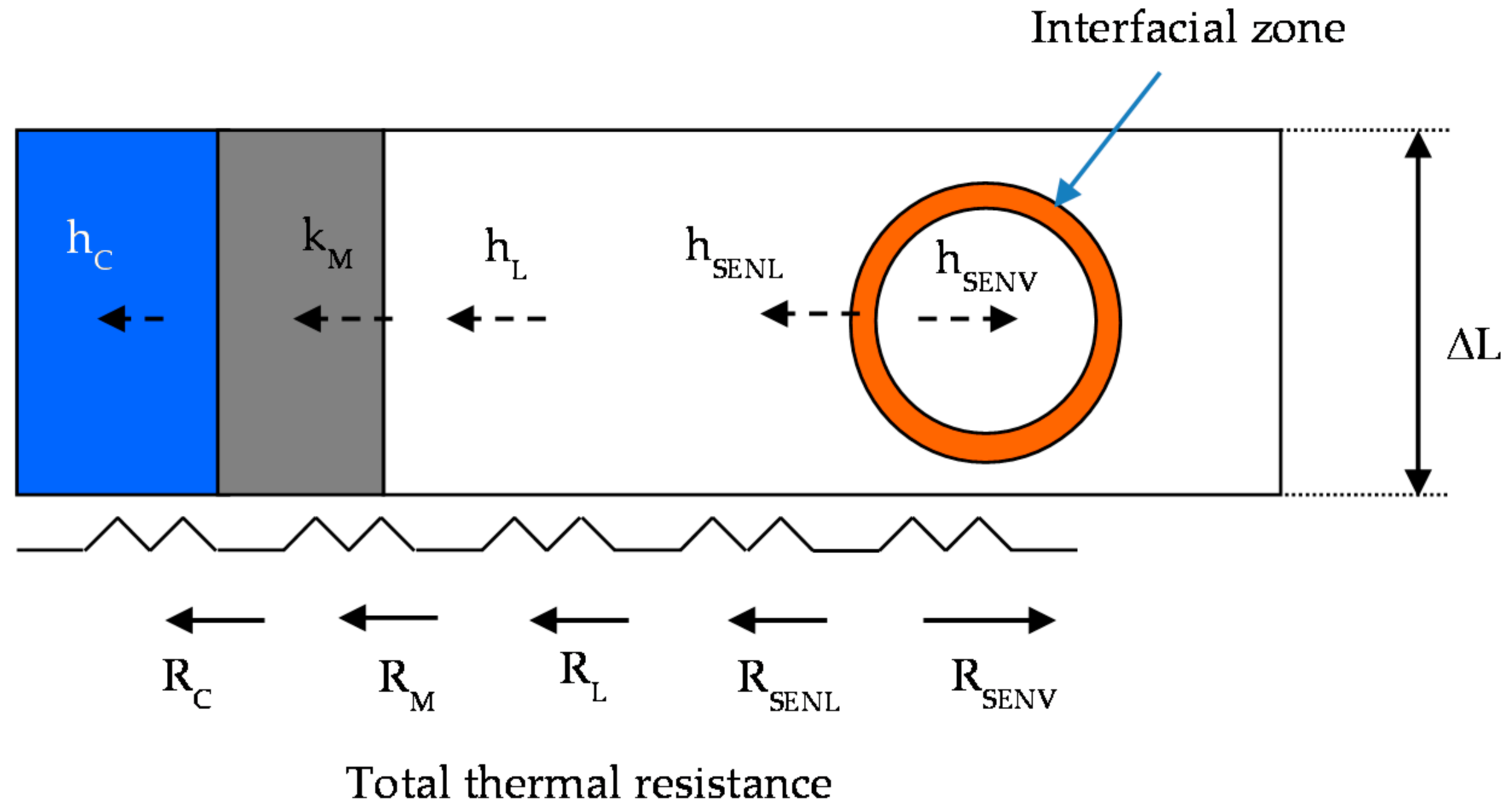
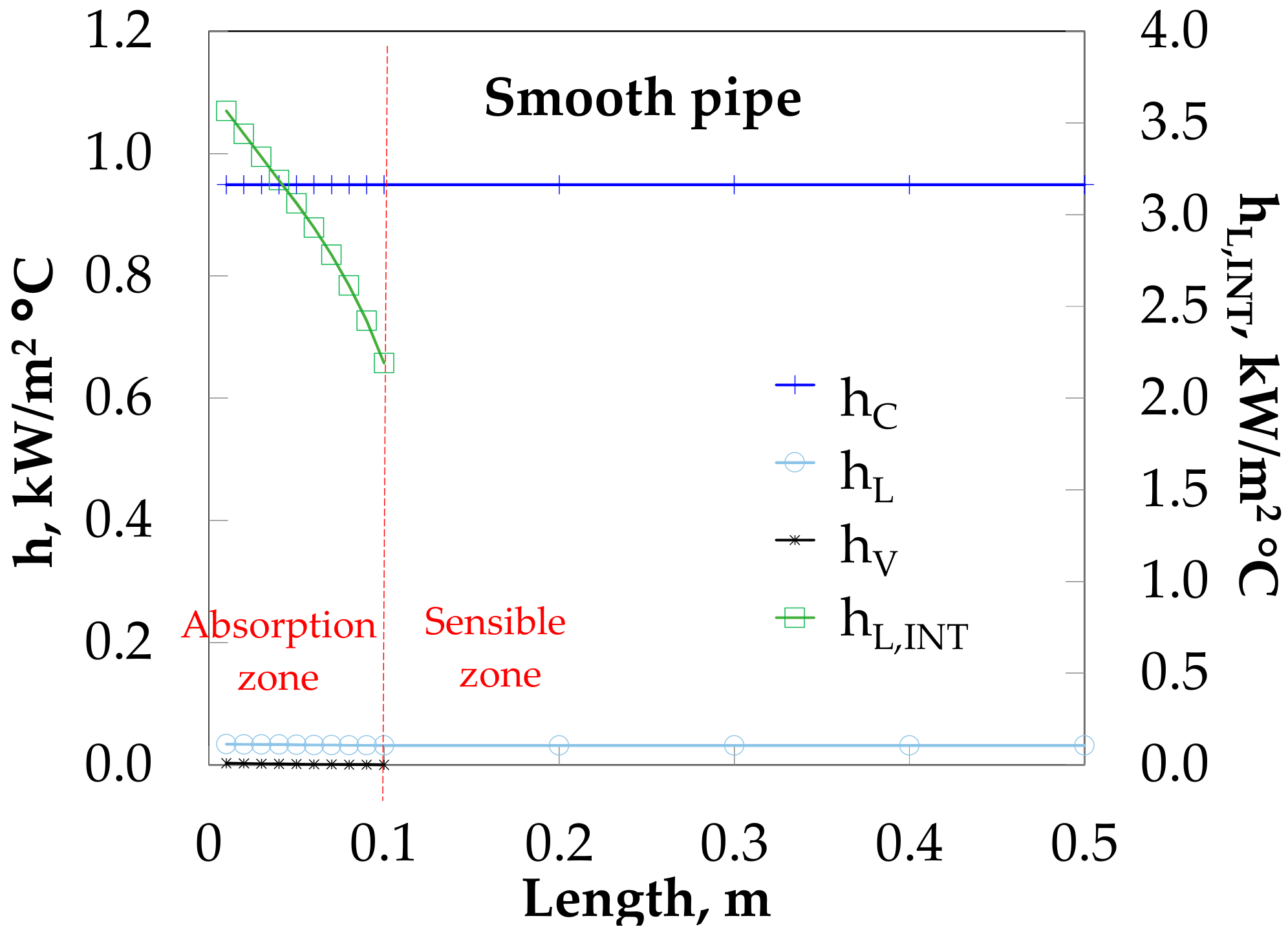
The difference between numerical and experimental data could be caused by the lower values of h
L at the absorption (
Table 4) than those of the sensible heat zone (
Table 3) because of the heat transfer equation (Equation (5)). The h
L was calculated by ΔT
ML in the sensible heat zone, but h
L was calculated by ΔT
ML,EQ equilibrium conditions in the absorption heat zone, because the results showed that the outlet solution temperature was higher than the inlet temperature input (due to the absorption heat). The use of ΔT
ML,EQ avoids this overlap of temperatures [
15] because solution temperatures are calculated from pressure, concentration and saturation conditions, however it includes energy not considered in the energy balance, then ΔT
ML,EQ obtains higher values than ΔT
ML. Mahmoud et al. [
23] presented an alternative driving potential for heat transfer defined as ΔT = T
L,IN,EQ − T
C,IN. It was also considered, but similar results were obtained.
A better theoretical temperature for the absorption process is required, in order to improve its agreement with the experimental data.
6. Conclusions
A theoretical and experimental comparison of an NH3-H2O bubble absorber was carried out with an HM in both, the solution and cooling water sides, in order to increase the absorber performance using a double tube heat exchanger. The following conclusions were drawn from this study:
A mathematical model was carried out in order to analyze the behavior of the absorber process with an SP and an HM using empirical correlations in the inside tube. The results showed that the absorption heat value was higher with the HM than the SP; however, simulation results were much lower than experimental data.
The experimental test consisted of comparing the absorption performance using an SP and an HM. The QAB,AVE was 1.17 and 1.40 kW/m2 for SP and HM insert without absorption (no injection of vapor). The QAB,AVE was 3.18 and 4.65 kW/m2 for SP and HM with absorption, respectively, this represented an increment of 31.61%.
Numerical results were analyzed using experimental data. It was shown that the absorption zone length was around 0.11 m for SP and HM, after that the heat transferred is only sensible heat. The difference of absorption heat load between numerical results was 28.0 and 21.9% lower than experimental data for SP and HM, respectively. It was caused by the lower value of hL in the absorption zone than sensible heat zone. The result could be improved using a log mean temperature difference equation (ΔTML) that includes the absorption heat.