Biodiesel Production from Bombacopsis glabra Oil by Methyl Transesterification Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Parameters of Degummed Oil
2.2. Biodiesel Analysis by TLC, 1H-NMR and GC
2.3. Analysis in Infrared Region
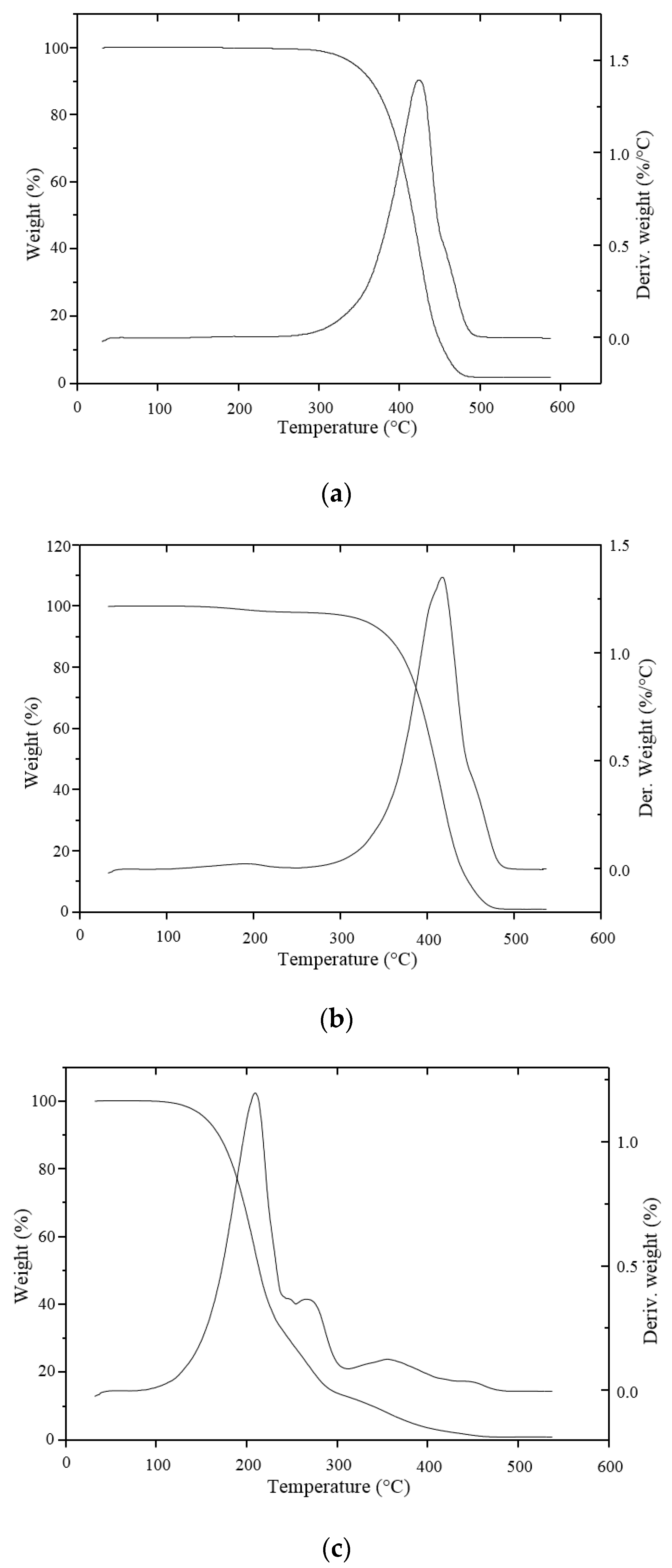
2.4. Thermogravimetric Analysis
2.5. Physico-Chemical Parameters
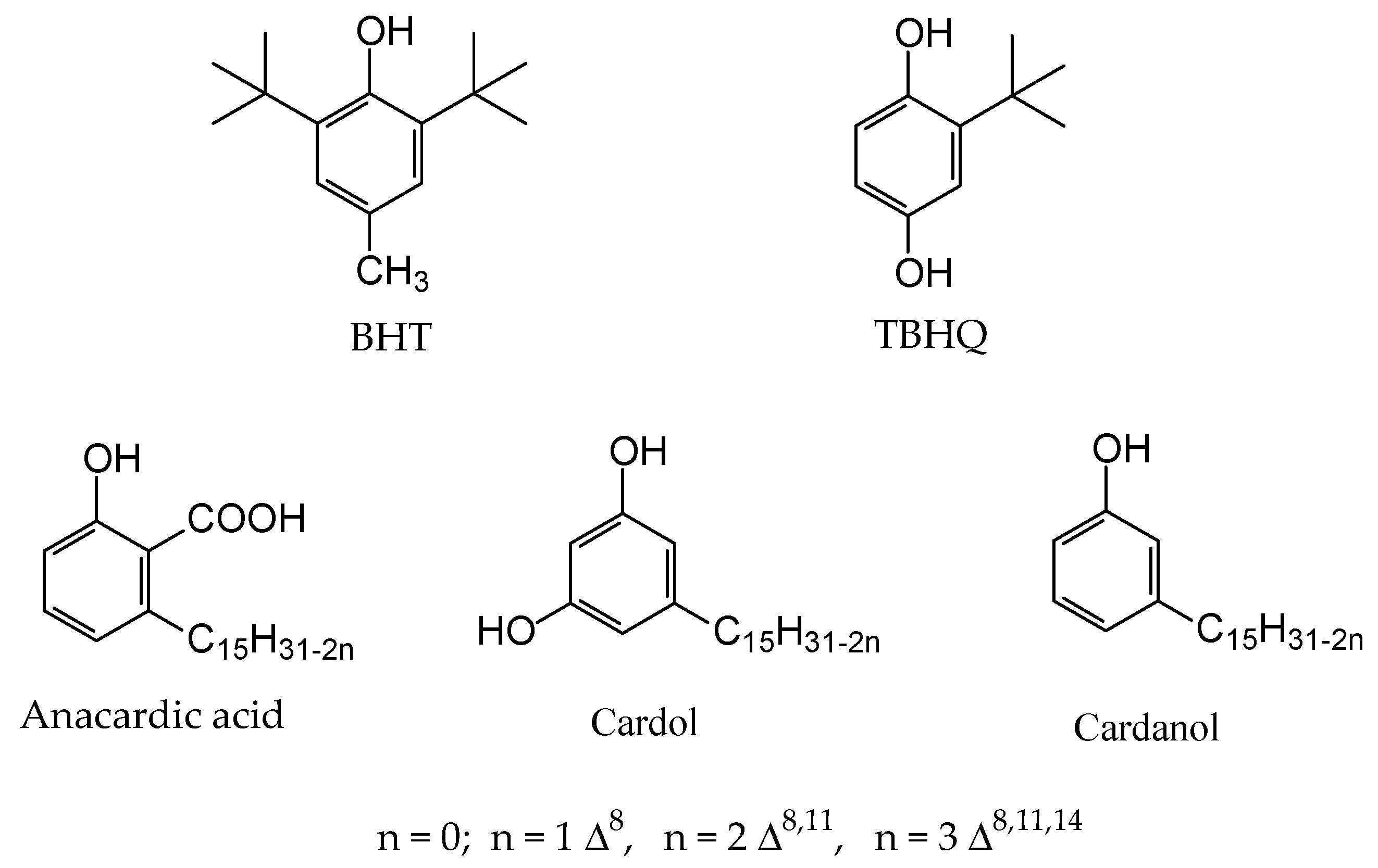
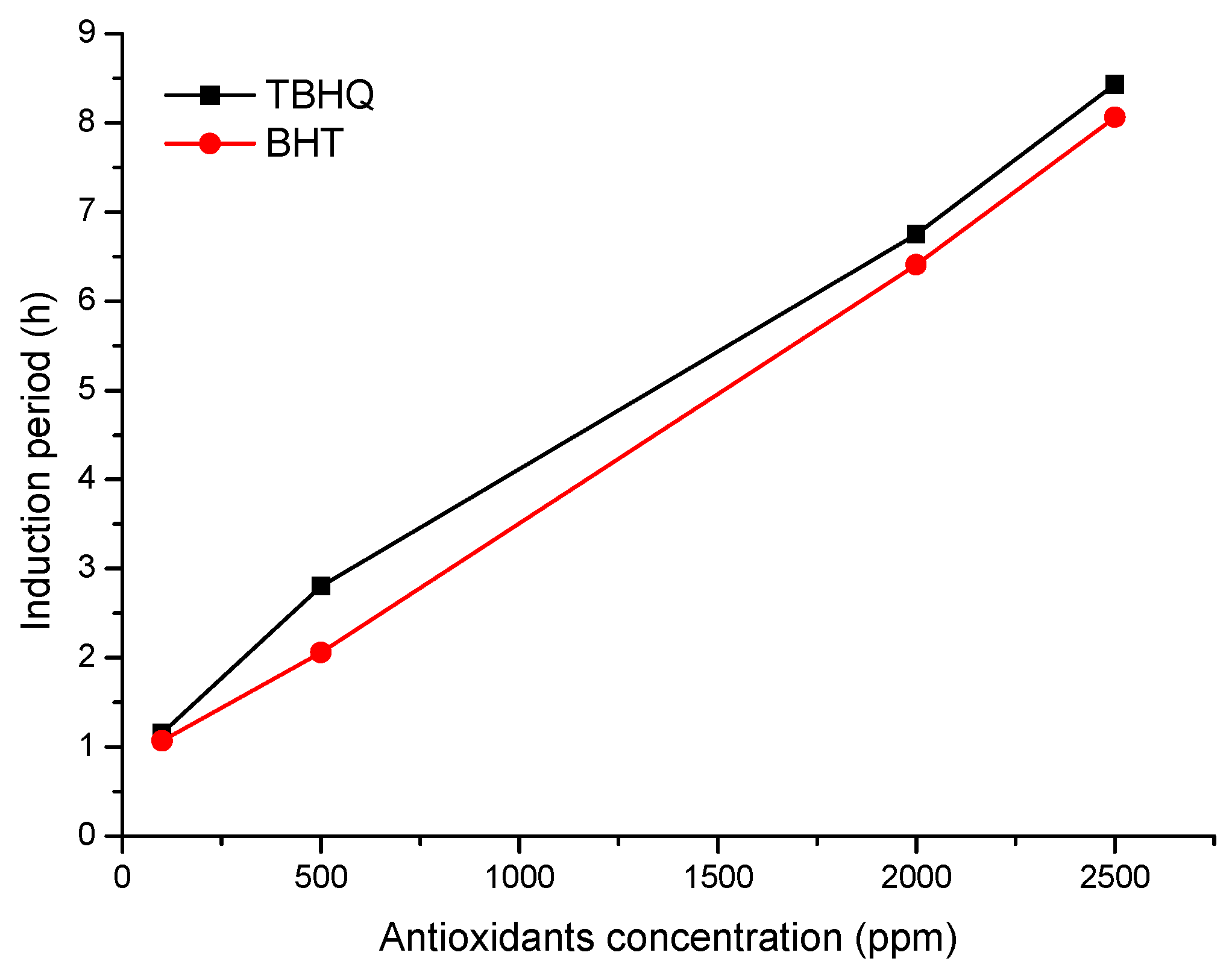
2.6. Addition Effect of Antioxidants in Stability from Methyl Biodiesel of B. glabra
3. Materials and Methods
3.1. Instrumentation
3.2. Plant Material
3.3. Extraction and Degumming of Oil for Obtaining Biodiesel
3.4. Synthesis of Biodiesel, TLC and 1H-NMR Analysis
3.5. Physicochemical Characterization, Infrared and Thermogravimetric Analysis of Biodiesel
3.6. Tests for Oxidation Stability
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lima, J.R.O.; Silva, R.B.; Silva, C.C.M.; Santos, L.S.S.; Santos, J.R., Jr.; Moura, E.M.; Moura, C.V.R. Biodiesel de babaçu (Orbignya sp.) obtido por via etanólica. Quim. Nova 2007, 30, 600–603. [Google Scholar] [CrossRef]
- Rinaldi, R.; Garcia, C.; Marciniuk, A.V.R.; Schuchardt, U. Síntese de biodiesel: Uma proposta contextualizada de experimento para laboratório de Química geral. Quim. Nova 2007, 30, 1364–1380. [Google Scholar] [CrossRef]
- Pereira, R.G.; Oliveira, C.D.; Oliveira, J.L.; Oliveira, P.C.P.; Fellows, C.E.; Piamba, O.E. Exhaust emissions and electric energy generation in a stationary engine using blends of biodiesel and soybean biodiesel. Renew. Energy 2007, 32, 2453–2460. [Google Scholar] [CrossRef]
- Paula, V.F.; Barbosa, L.C.A.; Errington, W.; Howard, O.W.; Cruz, M.P. Chemical constituents from Bombacopsis glabra (Pasq) A. Robyns: Complete 1H and 13C NMR assignments and X ray structure of 5-hidroxy-3,6,7,8,4′-pentamethoxyflavone. J. Braz. Chem. Soc. 2002, 13, 276–280. [Google Scholar] [CrossRef]
- Nyffeler, R.; Bayen, C.; Alverson, W.S.; Yen, A.; Whitlock, B.A.; Chase, M.W.; Baum, D.A. Phylogenetic analysis of the Malvadendrina clade (Malvaceae s.l.) based on plastid DNA sequences. Org. Divers. Evol. 2005, 5, 109–123. [Google Scholar] [CrossRef]
- Pospíšil, F.; Hrachová, B. Bombacopsis glabra (Pasq.) Robyns: A promising oil-bearing crop for the Socialist Republic of Vietnam. Agric. Trop. Subtrop. 1987, 20, 127–142. [Google Scholar]
- Chaves, M.H.; Araújo, F.D.S.; Moura, C.V.R.; Tozetto, L.J.; Aued-Pimentel, S.; Caruso, M.S.F. Chemical Characterization and Stability of the Bombacopsis glabra Nut Oil. Food Public Health 2012, 2, 104. [Google Scholar] [CrossRef]
- Scalon, S.P.Q.; Mussury, R.M.; Rigoni, M.R.; Scalon Filho, H. Crescimento inicial de mudas de Bombacopsis glabra (Pasq.) A. Robyns sob condição de sombreamento. Rev. Árvore 2003, 27, 753–758. [Google Scholar] [CrossRef]
- Piccolo, A.L.G. Sobre o fruto, semente e estágios iniciais de desenvolvimento de Bombacopsis glabra (Pasq.) A. Robyns. Sér. Bot. 1981, 5, 1–4. [Google Scholar]
- Breyne, H. Bombacopsis glabra (Pasquale) A. Robyns (Bombacaceae) espèce utile pour l’élevage et pour l’alimentation humaine. Tropicultura 1983, 1, 78–85. [Google Scholar]
- Vickery, J.R. The fatty acid composition of seed oils from ten plant families with particular reference to cyclopropene and dihydrosterculic acids. J. Am. Oil Chem. Soc. 1980, 57, 87–91. [Google Scholar] [CrossRef]
- Chaves, M.H.; Barbosa, A.S.; Moita Neto, J.M.; Aued-Pimentel, S.; Lago, J.H.G. Caracterização química do óleo da amêndoa de Sterculia striata St. Hil. et Naud. Quim. Nova 2004, 27, 404–408. [Google Scholar] [CrossRef]
- Aued-Pimentel, S.; Lago, J.H.G.; Chaves, M.H.; Kumagai, E.E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Nauds seed oil. J. Chromatogr. A 2004, 1054, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3nd ed.; Jonh Wiley and Sons: New York, NY, USA, 2009. [Google Scholar]
- Gomes Filho, J.C.; Peiter, A.S.; Pimentel, W.R.O.; Soletti, J.I.; Carvalho, S.H.V.; Meili, L. Biodiesel production from Sterculia striata oil by ethyl transesterification method. Ind. Crops Prod. 2015, 74, 767–772. [Google Scholar] [CrossRef]
- Cornelius, J.A.; Hammonds, T.W.; Shone, G.G. The composition of Bombacopsis glabra seed oil. J. Sci. Food Agric. 1965, 16, 170–172. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Nik Norulaini, N.A.; Mohd Omar, A.K.; Smith, R.L., Jr. Blending of supercritical carbon dioxide (SC-CO2) extracted palm kernel oil fractions and palm oil to obtain cocoa butter replacers. J. Food Eng. 2007, 78, 1397–1409. [Google Scholar] [CrossRef]
- Tangsathitkulchai, C.; Sittichaitaweekul, Y.; Tangsathitkulchai, M. Temperature Effect on the Viscosities of Palm Oil and Coconut Oil Blended with Diesel Oil. J. Am. Oil Chem. Soc. 2004, 81, 401–405. [Google Scholar] [CrossRef]
- Giannelos, P.N.; Zannikos, F.; Stournas, S.; Lois, E.; Anastopoulos, G. Tobacco seed oil as an alternative diesel fuel: Physical and chemical properties. Ind. Crops Prod. 2002, 16, 1–9. [Google Scholar] [CrossRef]
- Conceição, M.M.; Candeia, R.A.; Silva, F.C.; Bezerra, A.F.; Fernandes, V.J.; Souza, A.G. Thermoanalytical characterization of castor oil biodiesel. Renew. Sustain. Energy Rev. 2007, 11, 964–975. [Google Scholar] [CrossRef]
- Dermibaş, A. A direct route to the calculation of heating values of liquid fuels by using their density and viscosity measurements. Energy Convers. Manag. 2000, 41, 1609–1614. [Google Scholar]
- Geris, R.; Santos, N.A.C.; Amaral, B.A.; Maia, I.S.; Castro, V.D.; Carvalho, J.R.M. Biodiesel de soja—Reação de transesterificação para aulas práticas de Química Orgânica. Quim. Nova 2007, 30, 1369–1373. [Google Scholar] [CrossRef]
- RESOLUÇÃO ANP Nº 45, de 25.8.2014–DOU 26.8.2014. Available online: http://nxt.anp.gov.br/nxt/gateway.dll/leg/resolucoes_anp/2014/agosto/ranp%2045%20-%202014.xml (accessed on January 2016).
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Identificação Espectrométrica de Compostos Orgânicos, 7th ed.; LTC: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Goodrum, J.W. Volatility and boiling points of biodiesel from vegetable oils and tallow. Biomass Bioenergy 2002, 22, 205–211. [Google Scholar] [CrossRef]
- Goodrum, J.W.; Geller, D.P. Rapid thermogravimetric measurements of boiling points and vapor pressure of saturated medium- and long-chain triglycerides. Bioresour. Technol. 2002, 84, 75–80. [Google Scholar] [CrossRef]
- Moretto, E.; Fett, R. Tecnologia de Óleos e Gorduras Vegetais na Indústria de Alimento; Livraria Varela: São Paulo, Brazil, 1998. [Google Scholar]
- Araújo, F.D.S.; Araújo, I.C.; Costa, I.C.G.; Moura, C.V.R.; Chaves, M.H.; Araújo, E.C.E. Study of degumming process and evaluation of oxidative stability of methyl and ethyl biodiesel of Jatropha curcas L. oil from three different Brazilian states. Renew. Energy 2014, 71, 495–501. [Google Scholar] [CrossRef]
- Domingos, A.K.; Saad, E.B.; Vechiatto, W.W.D.; Wilhelm, H.M.; Ramos, L.P. The influence of BHA e TBHQ on the oxidation stability of soybean oil ethyl esters (biodiesel). J. Braz. Chem. Soc. 2007, 18, 416–423. [Google Scholar] [CrossRef]
- Ramalho, V.C.; Jorge, N. Antioxidantes utilizados em óleos, gorduras e alimentos gordurosos. Quim. Nova 2006, 29, 755–760. [Google Scholar] [CrossRef]
- Dunn, R.O. Effect of antioxidants on the oxidative stability of methyl soyate (biodiesel). Fuel Process. Technol. 2005, 86, 1017–1085. [Google Scholar] [CrossRef]
- Liang, Y.C.; May, C.Y.; Foon, C.S.; Ngan, M.A.; Hock, C.C.; Basiron, Y. The effect of natural and synthetic antioxidants on the oxidative stability of palm diesel. Fuel 2006, 85, 867–870. [Google Scholar] [CrossRef]
- Loh, S.L.; Chew, S.M.; Choo, Y.M. Oxidative stability and storage behavior of fatty methyl esters derived from used palm oil. J. Am. Oil Chem. Soc. 2006, 83, 947–952. [Google Scholar] [CrossRef]
- Sarin, R.; Sharma, M.; Sinharay, S.; Malhotra, R.K. Jatropha-palm biodiesel blends: An optimum mix for Asia. Fuel 2007, 86, 1365–1371. [Google Scholar] [CrossRef]
- Silva, M.C.D.; Conceição, M.M.; Fernandes, V.J., Jr.; Santos, I.M.G.; Souza, A.G. Avaliação do efeito antioxidante do líquido da castanha de caju (LCC) em óleo e biodiesel de mamona. In Congresso da Rede Brasileira de Tecnologia de Biodiesel; Anais; MCT/ABIPTI: Brasília, Brazil, 2006; Volume 1, pp. 192–195. [Google Scholar]
- Instituto Adolfo Lutz. Normas Analíticas do Instituto Adolfo Lutz. Métodos Químicos e Físicos Para Análise de Alimentos, 3rd ed.; IMESP: São Paulo, Brazil, 1985.
- Kumar, M.S.; Ramesh, A.; Nagalingam, B. An experimental comparison of methods to use methanol and Jatropha oil in a compression ignition engine. Biomass Bioenergy 2003, 25, 309–318. [Google Scholar] [CrossRef]
- Vicente, G.; Martinez, M.; Aracil, J. Optimisation of integrated biodiesel production. Part I. A study of the biodiesel purity and yield. Bioresour. Technol. 2006, 98, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Meher, L.C.; Sagar, D.V.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Antoniassi, R. Métodos de avaliação da estabilidade oxidativa de óleos e gorduras; The Center for Ethics, Philosophy and Public Affairs (CEPPA): Curitiba, Brazil, 2001; Volume 19, pp. 353–380. [Google Scholar]
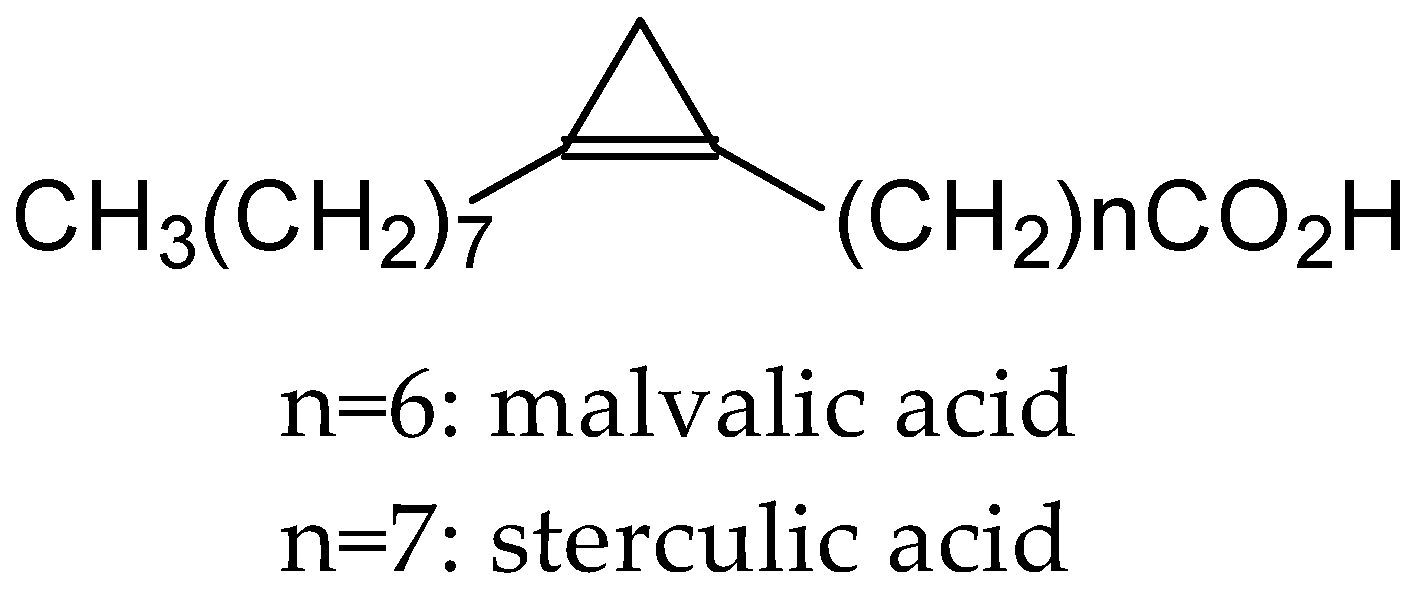





| Parameters | Value |
|---|---|
| Acidity level (mg KOH/g oil) | 0.5154 ± 0.0130 |
| Index of saponification (mg KOH/g oil) | 184.21 ± 0.98 |
| Viscosity 40.0 ± 0.1 °C (mm2/s) | 48.63 ± 0.02 |
| Characteristic | Unit | Methods | Results | Limit ANP [23] |
|---|---|---|---|---|
| Aspect | - | - | CFI | CFI |
| Specific mass at 20 °C | kg/m3 | ASTM D 4052 | 880.0 | 850–900 |
| Kinematic viscosity at 40 °C, max. | mm2/s | ASTM D 445 | 4.82 | 3–6 |
| Water and sediments, max. | % volume | ASTM D 2709 | 0.01 | 0.050 |
| Flash Point, min. | °C | ASTM D 93 | 172 | 100.0 |
| Copper corrosion, 3 h 50 °C, max. | - | ASTM 130 | 1b | 1 |
| Filter plugging point at cold, max. | °C | ASTM D 6371 | 2.0 | 19 |
| Acid value, max. | mg KOH/g | IAL | 0.03 | 0.50 |
| Free Glycerol, max. | % mass | ASTM D 6584 | 0.001 | 0.02 |
| Total Glycerol, max. | % mass | ASTM D 6584 | 0.029 | 0.25 |
| Monoacylglycerol, max. | % mass | ASTM D 6584 | 0.028 | 0.70 |
| Diacylglycerol, max. | % mass | ASTM D 6584 | 0.00 | 0.20 |
| Triacylglycerol, max. | % mass | ASTM D 6584 | 0.00 | 0.20 |
| Methanol, max. | % mass | EN 41110 | 0.010 | 0.20 |
| Oxidation stability at 110 °C, min. | h | EN 14112 | 0.76 | 8 |
| Concentration (ppm) | Induction Period (h) | |||
|---|---|---|---|---|
| TBHQ | BHT | CNSL | MBBg | |
| 0 | - | - | - | 0.76 |
| 100 | 1.16 | 1.07 | - | - |
| 500 | 2.80 | 2.06 | - | - |
| 2000 | 6.75 | 6.41 | - | - |
| 2500 | 8.43 | 8.06 | - | - |
| 5000 | - | - | 1.18 | - |
| 10,000 | - | - | 1.38 | - |
| 100,000 | - | - | 3.04 | - |
| 200,000 | - | - | 3.44 | - |
| 500,000 | - | - | 3.73 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva Araújo, F.D.; Do Nascimento Cavalcante, A.; Sousa, M.d.D.B.; De Moura, C.V.R.; Chaves, M.H.; Aued-Pimentel, S.; Fernandes Caruso, M.S.; Tozetto, L.J.; Kaline Morais Chaves, S. Biodiesel Production from Bombacopsis glabra Oil by Methyl Transesterification Method. Energies 2017, 10, 1360. https://doi.org/10.3390/en10091360
Da Silva Araújo FD, Do Nascimento Cavalcante A, Sousa MdDB, De Moura CVR, Chaves MH, Aued-Pimentel S, Fernandes Caruso MS, Tozetto LJ, Kaline Morais Chaves S. Biodiesel Production from Bombacopsis glabra Oil by Methyl Transesterification Method. Energies. 2017; 10(9):1360. https://doi.org/10.3390/en10091360
Chicago/Turabian StyleDa Silva Araújo, Francisca Diana, Antonio Do Nascimento Cavalcante, Maria das Dores B. Sousa, Carla Verônica Rodarte De Moura, Mariana Helena Chaves, Sabria Aued-Pimentel, Miriam Solange Fernandes Caruso, Luimar José Tozetto, and Soane Kaline Morais Chaves. 2017. "Biodiesel Production from Bombacopsis glabra Oil by Methyl Transesterification Method" Energies 10, no. 9: 1360. https://doi.org/10.3390/en10091360




