Encapsulated Nitrates Phase Change Material Selection for Use as Thermal Storage and Heat Transfer Materials at High Temperature in Concentrated Solar Power Plants
Abstract
1. Introduction
2. Phase Change Materials
3. EPCM State of the Art
- (1)
- Cascade system is a TES technique characterized by using encapsulated salts in packed beds, which are progressively ordered according to their melting points from the bottom to the top of a single tank [38]. This method allows one to significantly increase the heat transfer rate, due to the constant melting. In fact, cascades of macro-encapsulated PCMs can store 50% more energy per unit volume than conventional two tanks system of CSP plants [39]. Compared to traditional CSP two-tank systems, the cost of such a TES system could decrease from 27 to 16 $USD/kWh, according to [40,41].
- (2)
- Micro-encapsulated phase change slurry (MEPCS), which consists of a suspension where PCMs are microencapsulated, enhancing heat transfer due to direct surface contact between EPCM and heat transfer fluid (HTF), without altering the physical properties of the liquid (density, viscosity) [42].
- (3)
- Fluidized bed storage (FBS) is similar to the packed bed of cascade system, but instead of fixed PCM, EPCMs flow inside a TES single-tank. This method allows one to work at uniform temperature and increase the heat transfer rate of micro-encapsulated PCMs [43]. High heat transfer rates can be achieved between a fluidized bed of coated PCM particles and a heat exchanging surface [44]. In circulating fluidized bed, heat transfer rate determines heat exchange surface area required, which depends on capsule diameter [44,45].
4. Methodology
Criteria Selection for EPCM Materials
- Thermal: The melting point in within the desired operating temperature range. A high latent heat of fusion per mass unit, so that a smaller amount of material stores a given amount of energy. A high specific heat to provide additional significant sensible heat storage effects. A high thermal conductivity, so that the temperature gradients for charging and discharging the storage material are reduced.
- Physical: Small volume changes during phase transitions, so that a simple container and heat exchanger geometry can be used. High density. Exhibit little or no sub-cooling during freezing. Low vapor pressure in order to avoid stresses and problems with the container and the needed heat exchangers.
- Chemical: Chemical stability, no chemical decomposition and corrosion to construction materials. No phase separation. They must contain non-poisonous, non-flammable and non-explosive elements/compounds.
- Economical: Available in large quantities. Low cost.
5. Modeling of EPCM
5.1. Geometry Characterization
- (1)
- Geometry characterization 1: For the selections of materials, to compare in equal conditions different types of materials, R, r and T values are 1.1, 1.0 and 0.1 mm, respectively. These dimensions are selected considering previous studies under ideal conditions.
- (2)
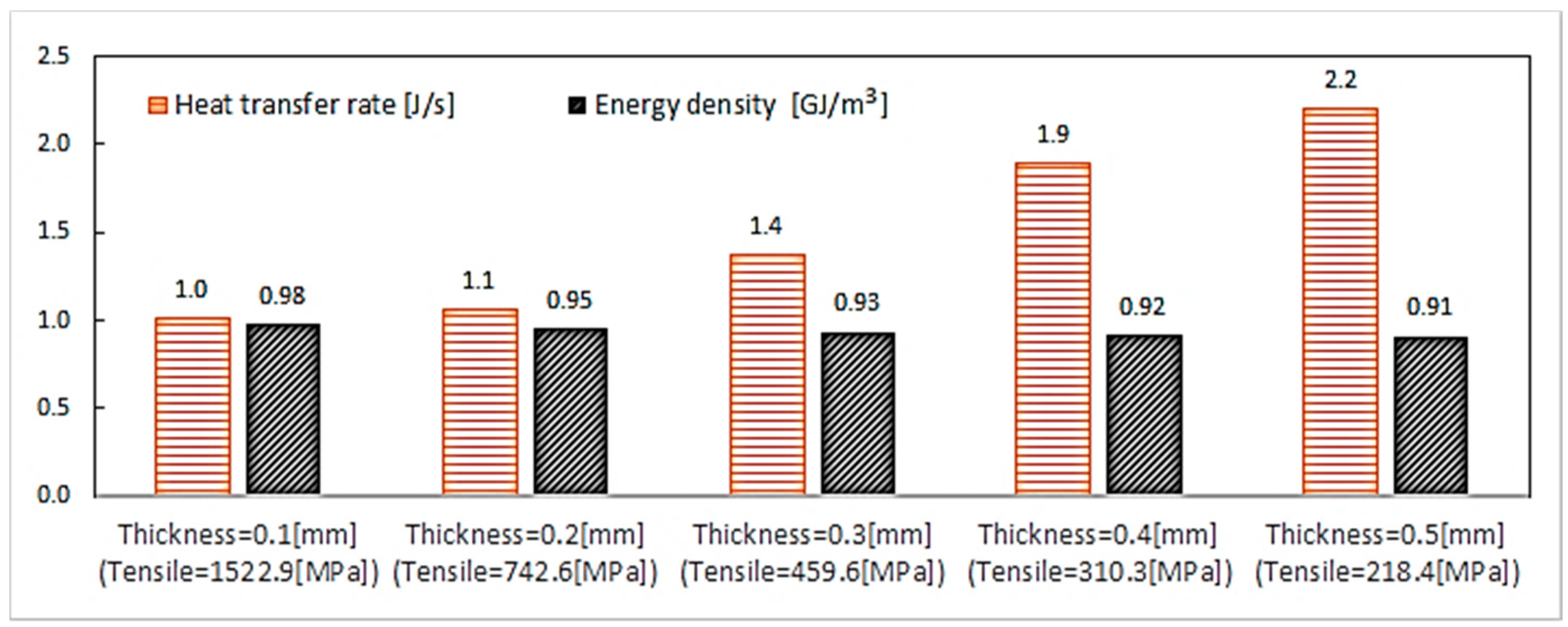
- Geometry characterization 2: Using the best material combination found, , in order to analyze the pressure and the thermal storage for different shell thickness for the thermomechanical coupling, the PCM thickness is varied five times ( 0.1, 0.2, 0.3, 0.4 and 0.5 mm) with R always equal to 1.1 mm and .
- (3)
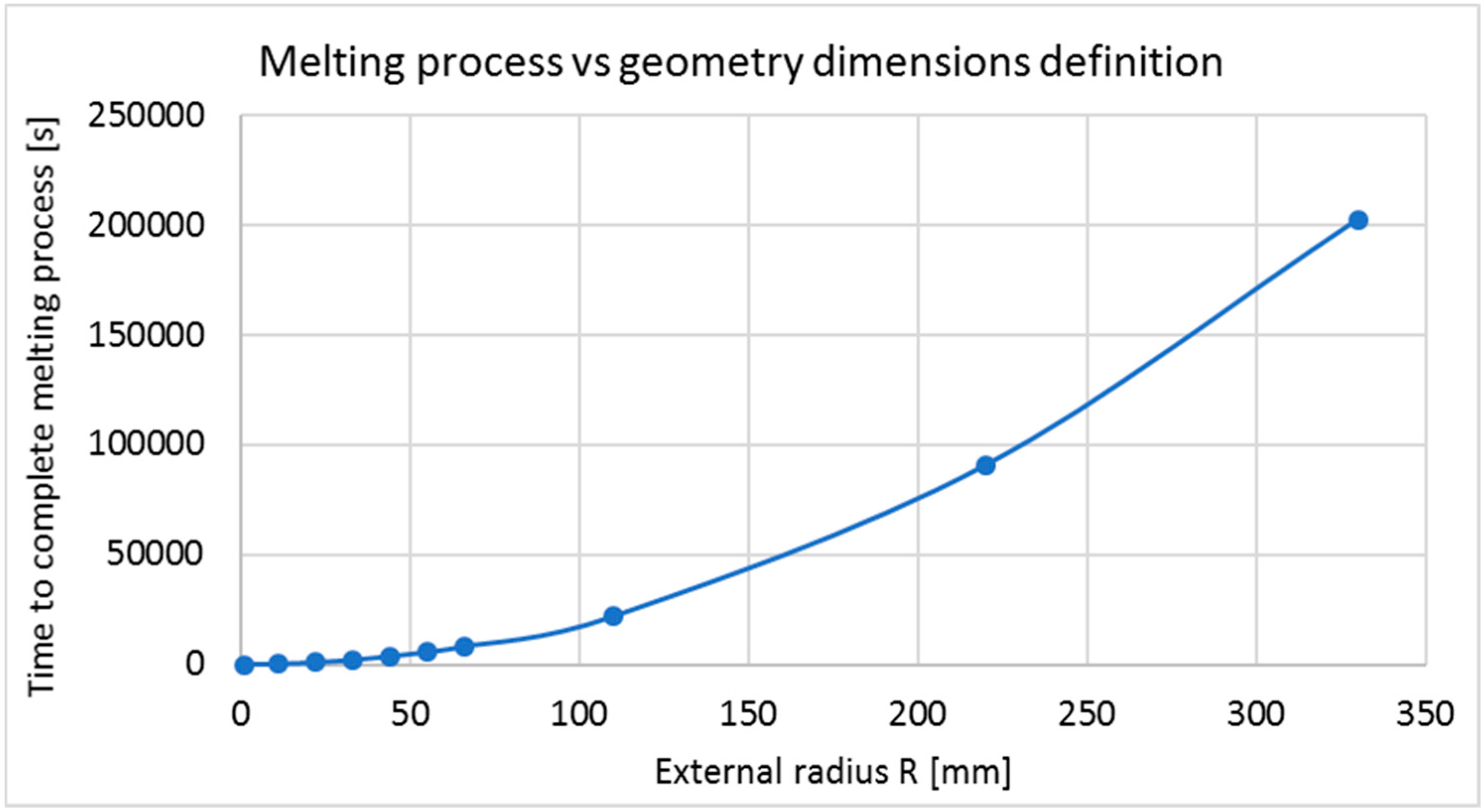
- Geometry characterization 3: Using the best materials combination for thermal analysis, R and r adopt several values, keeping the relative thickness ( equal to ). For this part the following dimensions in (mm) are used for = (1.1, 1.0), (11, 10), (22, 20), (33, 30), (55, 50), (66, 60), (77, 70), (110, 100), (220, 200), (330, 300). Since previous studies used different dimensions, making it difficult to decide a correct dimension, this evaluation is performed in order to know the effects of increasing geometry dimensions.
5.2. Heat Transfer Modeling
5.2.1. Shell Modeling
5.2.2. Shell/PCM Interface Modeling
5.2.3. PCM Modeling
6. Results
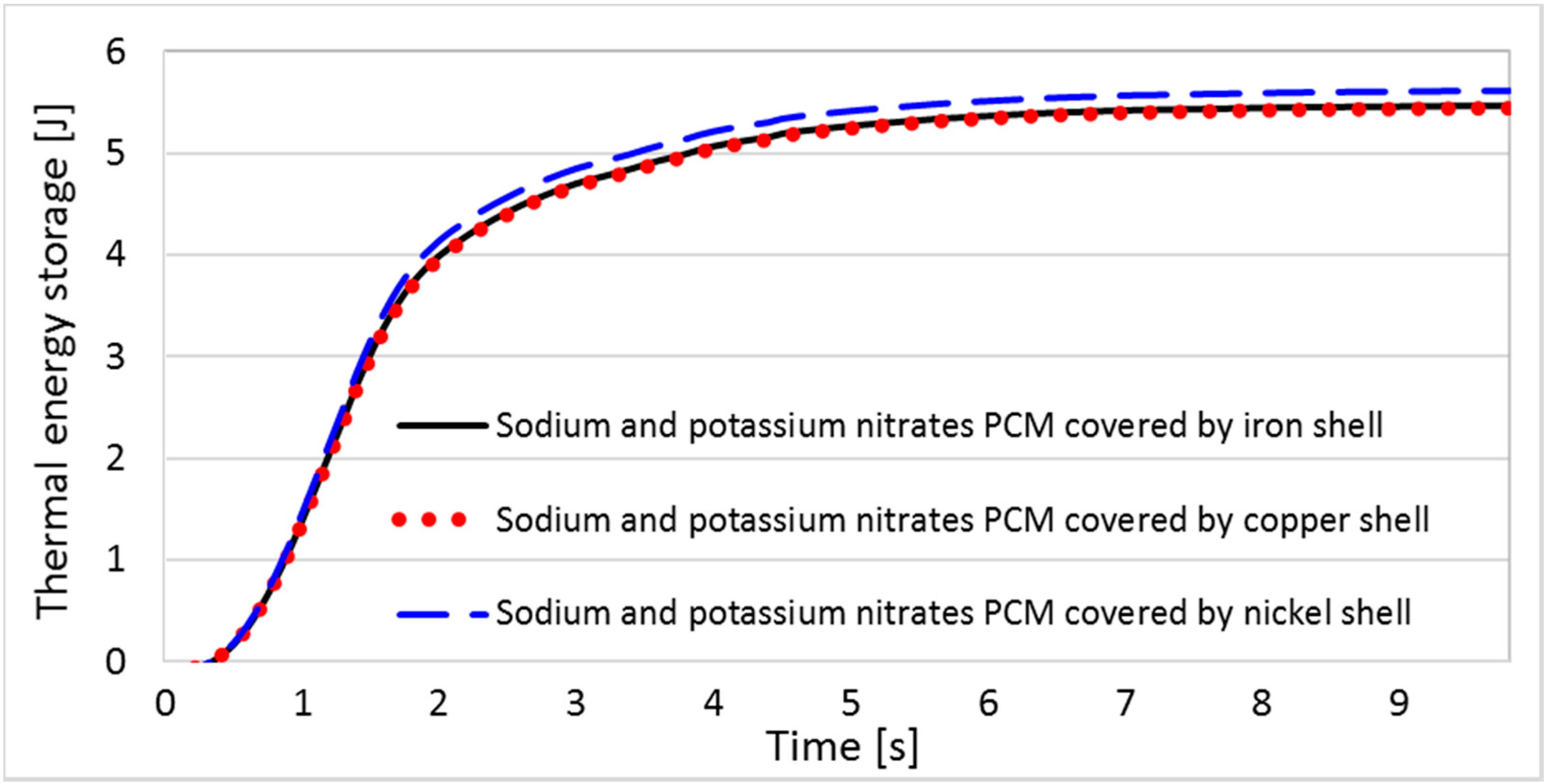
6.1. Results and Analyses of the EPCM Shell
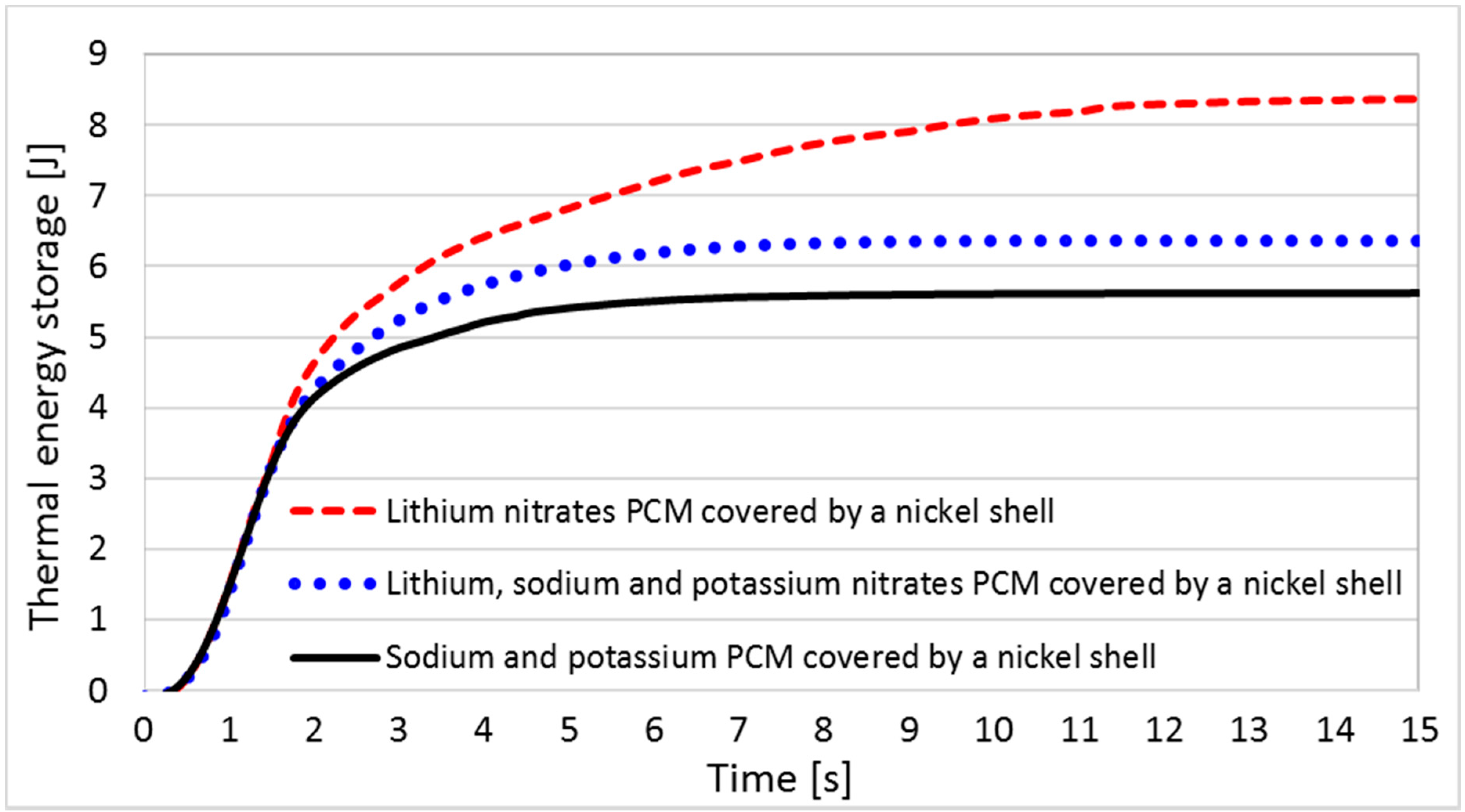
6.2. Results and Analyses of EPCMs (Geometry Characterization 1)
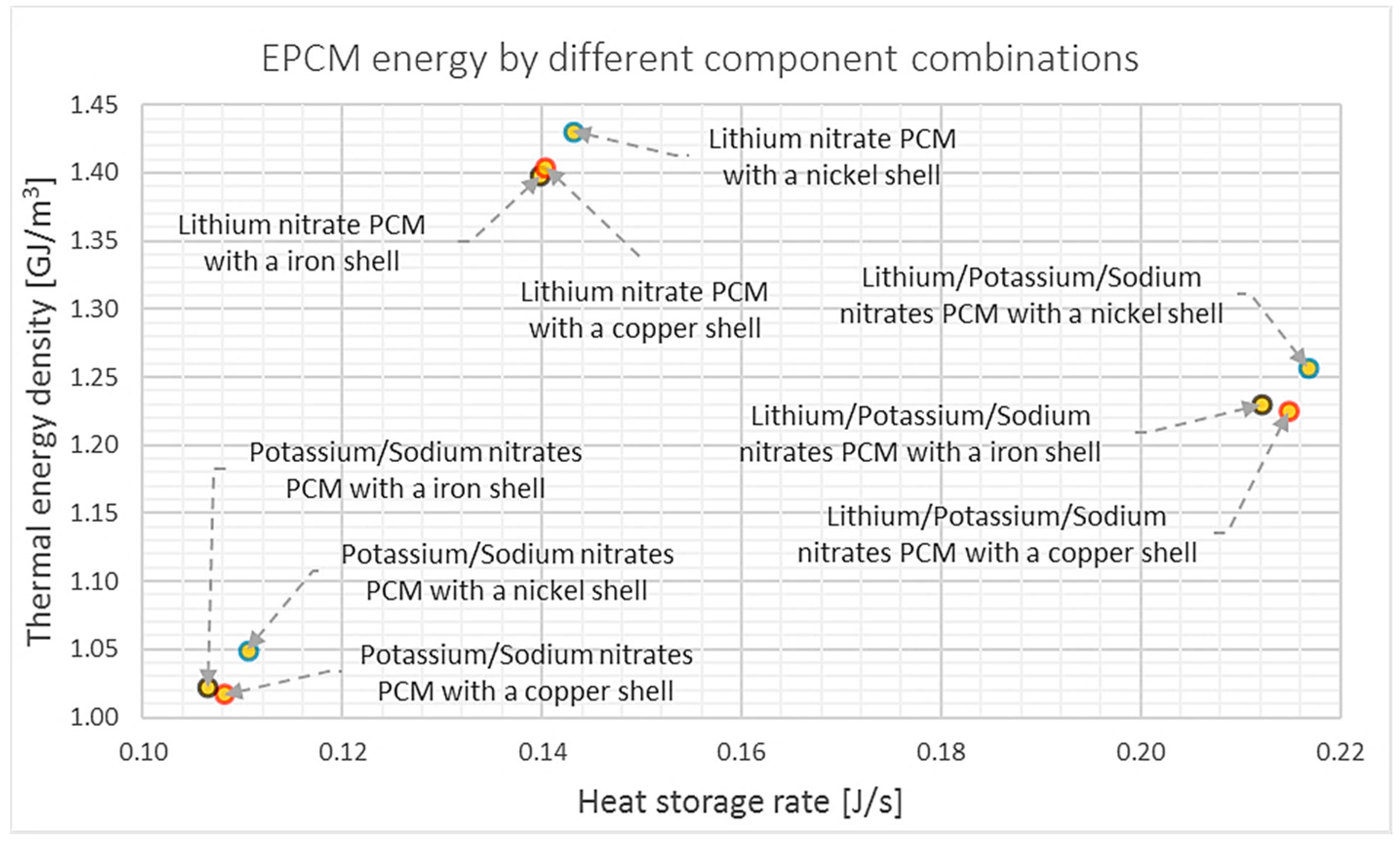
6.3. Results and Analyses of EPCM Dimensions Variation
6.3.1. Thickness Variation (Geometry Characterization 2)
6.3.2. Radius Variation (Geometry Characterization 3)
7. Conclusions
8. Future Works
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, Z.; Glatzmaier, G.; Mehos, M. Fluidized bed technology for concentrating solar power with thermal energy storage. J. Sol. Energy Eng. 2014, 136, 031014. [Google Scholar] [CrossRef]
- Alam, T.E.; Dhau, J.S.; Goswami, D.Y.; Stefanakos, E. Macroencapsulation and characterization of phase change materials for latent heat thermal energy storage systems. Appl. Energy 2015, 154, 92–101. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Fukahori, R.; Nomura, T.; Zhu, C.; Sheng, N.; Okinaka, N.; Akiyama, T. Macro-encapsulation of metallic phase change material using cylindrical-type ceramic containers for high-temperature thermal energy storage. Appl. Energy 2016, 170, 324–328. [Google Scholar] [CrossRef]
- U.S. Department of Energy. 2010 Solar Technologies Market Report; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2011.
- Green, A.; Diep, C.; Dunn, R.; Dent, J. High capacity factor CSP-PV hybrid systems. Energy Procedia 2015, 69, 2049–2059. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew. Sustain. Energy Rev. 2012, 16, 2118–2132. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Qian, T.; Guan, W.; Wang, X. Preparation and Characterization of KNO3/diatomite Shape-stabilized Composite Phase Change Material for High Temperature Thermal Energy Storage. J. Mater. Sci. Technol. 2017, 33, 198–203. [Google Scholar] [CrossRef]
- Cárdenas, B.; León, N. High temperature latent heat thermal energy storage: Phase change materials, design considerations and performance enhancement techniques. Renew. Sustain. Energy Rev. 2013, 27, 724–737. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.; Cabeza, L.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.S.; Zhang, S.D.; Tao, Q.H.; Pan, L.; Wang, Z.Y.; Zhang, Z.P.; Lei, Y.; Yang, S.K.; Zhao, H.P. In situ synthesis and phase change properties of Na2SO4 10H2O SiO2 solid nanobowls toward smart heat storage. J. Phys. Chem. C 2011, 115, 20061–20066. [Google Scholar] [CrossRef]
- Lopez, J.; Acem, Z.; Palomo Del Barrio, E. KNO3/NaNO3—Graphite materials for thermal energy storage at high temperature: Part II—Phase transition properties. Appl. Therm. Eng. 2010, 30, 1586–1593. [Google Scholar] [CrossRef]
- Wang, T.; Mantha, D.; Reddy, R.G. Thermal stability of the eutectic composition in LiNO3-NaNO3-KNO3 ternary system used for thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 100, 162–168. [Google Scholar] [CrossRef]
- Ping, W.; Harrowell, P.; Byrne, N.; Angell, C.A. Composition dependence of the solid state transitions in NaNO3/KNO3 mixtures. Thermochim. Acta 2009, 486, 27–31. [Google Scholar] [CrossRef]
- Sanusi, O.; Warzoha, R.; Fleischer, A.S. Energy storage and solidification of paraffin phase change material embedded with graphite nanofibers. Int. J. Heat Mass Transfer 2011, 54, 4429–4436. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, X.; Gao, C.; Bian, T.; Qiao, H.; Wei, Q. Structural morphology and thermal performance of composite phase change materials consisting of capric acid series fatty acid eutectics and electrospun polyamide6 nanofibers for thermal energy storage, Mate. Mater. Lett. 2012, 89, 43–46. [Google Scholar] [CrossRef]
- Cai, Y.; Song, L.; He, Q.; Yang, D.; Hu, Y. Preparation, thermal and flammability properties of a novel form-stable phase change materials based on high density polyethylene/poly(ethylene-co-vinyl acetate)/organophilic montmorillonite nanocomposites/paraffin. Energy Convers. Manag. 2008, 49, 2055–2062. [Google Scholar] [CrossRef]
- Feng, L.L.; Zheng, J.; Yang, H.Z.; Guo, Y.L.; Li, W.; Li, X.G. Preparation and characterization of polyethylene glycol/active carbon composites as shapestabilized phase change materials. Sol. Energy Mater. Sol. Cells 2010, 95, 644–650. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, T. Study on preparation and thermal properties of sodium nitrate/silica composite as shape-stabilized phase change material. Thermochim. Acta 2015, 613, 66–70. [Google Scholar] [CrossRef]
- Mills, A.; Farid, M.; Selman, J.R.; Al-Hallaj, S. Thermal conductivity enhancement of phase change materials using a graphite matrix. Appl. Therm. Eng. 2006, 26, 1652–1661. [Google Scholar] [CrossRef]
- Wang, Q.; Wong, T.J.; Xia, L. Preparation and thermal characterization of expanded graphite/paraffin composite phase change material. Carbon 2010, 48, 2538–2548. [Google Scholar]
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Tamme, T.; Bauer, J.; Buschle, D.; Müller-Steinhagen, H.; Steinmann, W.D. Latent heat storage above 120 °C for applications in the industrial process heat sector and solar power generation. Int. J. Energy Res. 2008, 32, 264–271. [Google Scholar] [CrossRef]
- Mao, A.; Park, J.H.; Han, G.Y.; Seo, T.; Kang, Y. Heat transfer characteristics of high temperature molten salt for storage of thermal energy. Korean J. Chem. Eng. 2010, 27, 1452–1457. [Google Scholar] [CrossRef]
- Chen, J.C.; Eichlberger, J.L. Encapsulated Phase Change Thermal Energy Storage Materials. U.S. Patent 4504402, 12 March 1985. [Google Scholar]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Jiang, Z.; Leng, G.; Ye, F.; Ge, Z.; Liu, C.; Wang, L.; Huang, Y.; Ding, Y. Form-stable LiNO3-NaNO3-KNO3-Ca(NO3)2/calcium silicate composite phase change material (PCM) for mid-low temperature thermal energy storage. Energy Convers. Manag. 2015, 106, 165–172. [Google Scholar] [CrossRef]
- Mathur, A.; Kasetty, R.; Oxley, J.; Mendez, J.; Nithyanandam, K. Using encapsulated phase change salts for concentrated solar power plant, for Solar PACES 2013. Energy Procedia 2014, 49, 908–915. [Google Scholar] [CrossRef]
- Jacob, R.; Bruno, F. Review on shell materials used in the encapsulation of phase change materials for high temperature thermal energy storage. Renew. Sustain. Energy Rev. 2015, 48, 79–87. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Deng, Y.; Guan, W.; Ning, L. Diatomite: A promising natural candidate as carrier material for low, middle and high temperature phase change material. Energy Convers. Manag. 2015, 98, 34–45. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Lopez, J.; Cáceres, G.; Palomo Del Barrio, E.; Jomaa, W. Confined melting in deformable porous media: A first attempt to explain the graphite/salt composites behavior. Int. J. Heat Mass Transfer 2010, 53, 1195–1207. [Google Scholar] [CrossRef]
- Blaney, J.J.; Neti, S.; Misiolek, W.Z.; Oztekin, A. Stresses in containment capsule for encapsulated phase change materials. Appl. Therm. Eng. 2013, 50, 555–561. [Google Scholar] [CrossRef]
- Blaney, J.J. Stresses in Containment Vessels for Encapsulated Phase Change Materials. Master’s Thesis, Lehigh University, Bethlehem, PA, USA, 2010. [Google Scholar]
- Mehling, H.; Cabeza, L. Phase change materials and their basic properties. Therm. Energy Storage Sustain. Energy Consum. 2007, 234, 257–277. [Google Scholar]
- Pitié, F.; Zhao, C.Y.; Cáceres, G. Thermo-mechanicalanalysis of ceramic encapsulated phase-change material (PCM) particles. Energy Environ. Sci. 2011, 4, 2117–2124. [Google Scholar] [CrossRef]
- Parrado, C.; Cáceres, G.; Bize, F.; Bubnovich, V.; Baeyens, J.; Degrève, J.; Zhang, H.L. Thermo-mechanical analysis of copper-encapsulated NaNO3–KNO3. Chem. Eng. Res. Des. 2015, 93, 224–231. [Google Scholar] [CrossRef]
- Zhao, W. Characterization of encapsulated phase change materials for thermal energy storage. Ph.D. Thesis, Lehigh University, Bethlehem, PA, USA, 2013. [Google Scholar]
- Zhang, H.; Baeyens, J.; Cáceres, G.; Degrève, J.; Lv, Y. Thermal energy storage: Recent developments and practical aspects. Prog. Energy Combust. Sci. 2016, 53, 1–40. [Google Scholar] [CrossRef]
- Thermal Energy Storage (TES) for Concentrating Solar Power (CSP). Available online: http://www.energy.ca.gov/research/notices/2012-08-23_workshop/presentations/3-1_Terrafore_CEC_Workshop.pdf (accessed on 29 August 2017).
- Flueckiger, S.M.; Yang, Z.; Garimella, S.V. Design of Molten-Salt Thermocline Tanks for Solar Thermal Energy Storage; CTRC Research Publications, Paper 191; Purdue University: West Lafayette, IN, USA, 2013. [Google Scholar]
- Chen, B.; Wang, X.; Zeng, R.; Zhang, Y.; Niu, J. An experimental study of convective heat transfer with microencapsulated phase change material suspension: Laminar flow in a circular tube under constant heat flux. Exp. Therm. Fluid Sci. 2008, 32, 1638–1646. [Google Scholar] [CrossRef]
- Izquierdo, M. Heat Transfer and tHermal Storage in Fixed and Fluidized Beds of Phase Change Material. Ph.D. Thesis, Universidad Carlos III de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Pitié, F.; Zhao, C.Y.; Baeyens, J.; Degrève, J.; Zhang, H.L. Circulating fluidized bed heat recovery/storage and its potential to use coated phase-change-material (PCM) particles. Appl. Energy 2013, 109, 505–513. [Google Scholar] [CrossRef]
- Peng, H.; Dong, H.; Ling, X. Thermal investigation of PCM-based high temperature thermal energy storage in packed bed. Energy Convers. Manag. 2014, 81, 420–427. [Google Scholar] [CrossRef]
- Lopez, J. Nouveaux Matériaux Graphite/Selpour le Stockage D’énergie à haute Température. Étude Despropriétés de Changement de Phase. Ph.D. Thesis, Université Bordeaux 1, Bordeaux, France, 2007. [Google Scholar]
- Mehling, H.; Hiebler, S.; Ziegler, F. Latent heat storage using a PCM-graphite composite material. In Proceedings of the 8th International Conference on Thermal Energy Storage, Stuttgart, Germany, 28 August–1 September 2000. [Google Scholar]
- Py, X.; Regis, O.; Sylvain, M. Paraffin/porous-graphite-matrix composite as a high and constant power thermal storage material. Int. J. Heat Mass Transf. 2001, 44, 2727–2737. [Google Scholar] [CrossRef]
- Bauer, C.; Wirtz, R.A. Thermal characteristics of a compact, passive thermal energy storage device. In Proceedings of the 2000 ASME IMECE, Orlando, FL, USA, 5–10 November 2000; pp. 1–7. [Google Scholar]
- Tong, X.; Khan, T.A.; Amin, M.R. Enhancement of heat transfer by inserting a metal matrix into a phase change material. Numer. Heat Transf. Part A Appl. 1996, 30, 125–141. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Preparation and thermal characterization of paraffin/metal foam composite phase change material. Appl. Energy 2013, 112, 1357–1366. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.G. Numerical study on the thermal behavior of phase change materials (PCMs) embedded in porous metal matrix. Sol. Energy 2014, 99, 172–184. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Lu, W.; Tian, Y. Heat transfer enhancement for thermal energy storage using metal foams embedded within phase change materials (PCMs). Sol. Energy 2010, 84, 1402–1412. [Google Scholar] [CrossRef]
- Vadwala, P. Thermal energy storage in metal foams filled with paraffin wax. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2011. [Google Scholar]
- Li, M. A nano-graphite/paraffin phase change material with high thermal conductivity. Appl. Energy 2013, 106, 25–30. [Google Scholar] [CrossRef]
- Mettawee, E.S.; Assassa, G.M.R. Thermal conductivity enhancement in a latent heat storage system. Sol. Energy 2007, 81, 839–845. [Google Scholar] [CrossRef]
- Zeng, J.L.; Sun, L.X.; Xu, F.; Tan, Z.C.; Zhang, Z.H.; Zhang, J.; Zhang, T. Study of a PCM based energy storage system containing Ag nanoparticles. J. Therm. Anal. Calorim. 2007, 87, 369–373. [Google Scholar] [CrossRef]
- Khodadadi, J.M.; Hosseinizadeh, S.F. Nanoparticle-enhanced phase change materials (NEPCM) with great potential for improved thermal energy storage. Int. Commun. Heat Mass Transf. 2007, 34, 534–543. [Google Scholar] [CrossRef]
- Velraj, R.; Seeniraj, R.V.; Hafner, B.; Faber, C.; Schwarzer, K. Heat transfer enhancement in a latent heat storage system. Sol. Energy 1999, 65, 171–180. [Google Scholar] [CrossRef]
- Ettouney, H.M.; Alatiqi, I.; Al-Sahali, M.; Al-Ali, S.A. Heat transfer enhancement by metal screens and metal spheres in phase change energy storage systems. Renew. Energy 2004, 29, 841–860. [Google Scholar] [CrossRef]
- Elgafy, A.; Lafdi, K. Effect of carbon nanofiber additives on thermal behavior of phase change materials. Carbon 2005, 43, 3067–3074. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press Inc.: Boca Raton, FL, USA, 2009. [Google Scholar]
- COMSOL. Multiphysics, version 5.2a; Virtual Library of Materials; COMSOL: Stockholm, Sweden, 2016.
- Sharma, N.K.; Misra, R.K.; Sharma, S. Modeling of thermal expansion behavior of densely packed Al/SiC composites. Int. J. Solids Struct. 2016, 102–103, 77–88. [Google Scholar] [CrossRef]
- Stadler, F.; Antrekowitsch, H.; Fragner, W.; Kaufmann, H.; Pinatel, E.R.; Uggowitzer, P.J. The effect of main alloying elements on the physical properties of Al–Si foundry alloys. Mater. Sci. Eng. A 2013, 560, 481–491. [Google Scholar] [CrossRef]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–402. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Jegadheeswaran, S.; Pohekar, S.D. Performance enhancement in latent heat thermal storage system: A review. Renew. Sustain. Energy Rev. 2009, 13, 2225–2244. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat transfer fluids for concentrating solar power systems—A review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Fossile, L.M. Comparison and simulation of salt-ceramic composites for use in high temperature concentrated solar power. Master’s Thesis, University of Nevada, Las Vegas, NV, USA, 2012. [Google Scholar]
- Lachheb, M.; Adili, A.; Albouchi, F.; Mzali, F.; Nasralla, S.B. Thermal properties improvement of Lithium nitrate/Graphite composite phase change materials. Appl. Therm. Eng. 2016, 102, 922–931. [Google Scholar] [CrossRef]
- Hübner, S.; Eck, M.; Stiller, C.; Seitz, M.; Hübner, S. Techno-economic heat transfer optimization of large scale latent heat energy storage systems in solar thermal power plants. Appl. Therm. Eng. 2016, 98, 483–491. [Google Scholar] [CrossRef]
- Janz, G.J.; Allen, C.B.; Bansal, N.P.; Murphy, R.M.; Tomkins, R.P.T. Physical Properties Data Compilations Relevant to Energy Storage. II. Molten Salts: Data on Single and Multi-Component Salt Systems; U.S. Department of Commerce, National Bureau of Standards: Gaithersburg, MD, USA, 1978.
- Nunes, V.M.B.; Queirós, C.S.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A. Molten salts as engineering fluids—A review Part I. Molten alkali nitrates. Appl. Energy 2016, 183, 603–611. [Google Scholar] [CrossRef]
- Zhang, H.L.; Baeyens, J.; Degrève, J.; Cáceres, G.; Segal, R.; Pitié, F. Latent heat storage with tubular-encapsulated phase change materials (PCMs). Energy 2014, 76, 66–72. [Google Scholar] [CrossRef]
- Williams, D.F. Assessment of Candidate Molten Salt Coolants for the NGNP/NHI Heat Transfer Loop; ORNL/TM-2006/69; Nuclear Science and Technology Division, Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2006.
- Neti, S. Novel Thermal Storage Technologies for Concentrating Solar Power Generation; Research Performance Progress Report; Lehigh University: Bethlehem, PA, USA, 2011. [Google Scholar]
- Janz, G.J. Thermodynamic and Transport Properties for Molten Salts: Correlation Equations for Critically Evaluated Density, Surface Tension, Electrical Conductance, and Viscosity Data; American Institute of Physics: Park City, MD, USA, 1988. [Google Scholar]
- Roget, F.; Favotto, C.; Rogez, J. Study of the KNO3–LiNO3 and KNO3–NaNO3–LiNO3 eutectics as phase change materials for thermal storage in a low-temperature solar power plant. Sol. Energy 2013, 95, 155–169. [Google Scholar] [CrossRef]
- Villada, C.; Bolívar, F.; Jaramillo, F.; Castaño, J.G.; Echeverría, F. Thermal evaluation of molten salts for solar thermal energy storage. Renew. Energy Power Qual. J. 2014, 1, 622–625. [Google Scholar] [CrossRef]
- Zheng, Y.; Barton, J.; Tuzla, K.; Chen, J.; Neti, S.; Oztekin, A.; Misiolek, W. Experimental and computational study of thermal energy storage with encapsulated NaNO3 for high temperature applications. Sol. Energy 2015, 115, 180–194. [Google Scholar] [CrossRef]
- Munoz-Sanchez, B.; Iparraguirre-Torres, I.; Madina-Arrese, V.; Izagirre-Etxeberria, U.; Unzurrunzaga-Iturbe, A.; Garcia-Romero, A. Encapsulated high temperature PCM as active filler material in a thermocline-based thermal storage system. Energy Procedia 2015, 69, 937–946. [Google Scholar] [CrossRef]
- Nomura, T.; Okinaka, N.; Akiyama, T. Technology of latent heat storage for high temperature application: A review. Iron Steel Inst. Jpn. 2010, 50, 1229–1239. [Google Scholar] [CrossRef]
- Cao, L.; Su, D.; Tang, Y.; Fang, G.; Tang, F. Properties evaluation and applications of thermal energy storage materials in buildings. Renew. Sustain. Energy Rev. 2015, 48, 500–522. [Google Scholar] [CrossRef]
- Alam, T. Experimental investigation of encapsulated phase change materials for thermal energy storage, and applications. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2015. [Google Scholar]
- COMSOL. Heat Transfer Module: User’s Guide, version 5.2a; COMSOL: Stockholm, Sweden, 2016. [Google Scholar]
- Nicoletto, G.; Riva, E.; Di Filippo, A. High Temperature Fatigue Behavior of Eutectic Al-Si-Alloys Used for Piston Production. Procedia Eng. 2014, 74, 157–160. [Google Scholar] [CrossRef]
- Ravi Chandran, K.S. A physical model and constitutive equations for complete characterization of S-N fatigue behavior of metals. Acta Mater. 2016, 121, 85–103. [Google Scholar] [CrossRef]
- Fatoba, O.; Akid, R. Low Cycle Fatigue Behaviour of API 5L X65 Pipeline Steel at Room Temperature. Procedia Eng. 2014, 74, 279–286. [Google Scholar] [CrossRef]
- Zhang, X.C.; Li, H.C.; Zeng, X.; Tu, S.T.; Zhang, C.C.; Wang, Q.Q. Fatigue behavior and bilinear Coffin-Manson plots of Ni-based GH4169 alloy with different volume fractions of δ phase. Mater. Sci. Eng. A 2017, 682, 12–22. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Z.J.; Zhang, P.; Zhang, Z.F. Extremely-low-cycle fatigue behaviors of Cu and Cu–Al alloys: Damage mechanisms and life prediction. Acta Mater. 2015, 83, 341–356. [Google Scholar] [CrossRef]
- Szusta, J.; Seweryn, A. Experimental study of the low-cycle fatigue life under multiaxial loading of aluminum alloy EN AW-2024-T3 at elevated temperatures. Int. J. Fatigue 2017, 96, 28–42. [Google Scholar] [CrossRef]
- Unigovski, Y.B.; Grinberg, A.; Gutman, E.M. Low-cycle Fatigue of the Light Advanced Materials. Procedia Eng. 2013, 66, 713–722. [Google Scholar] [CrossRef]
- Cáceres, G.; Montané, M.; Nasirov, S.; O’Ryan, R. Review of Thermal Materials for CSP Plants and LCOE Evaluation for Performance Improvement using Chilean Strategic Minerals: Lithium Salts and Copper Foams. Sustainability 2016, 8, 106. [Google Scholar] [CrossRef]
- Infomine. InvestmentMine: Mining Markets and Investments. Available online: http://www.infomine.com/investment/metal-prices/ (accessed on 1 July 2017).
- Cabeza, L.F. Advances in Thermal Energy Storage Systems: Methods and Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 22–63. [Google Scholar]
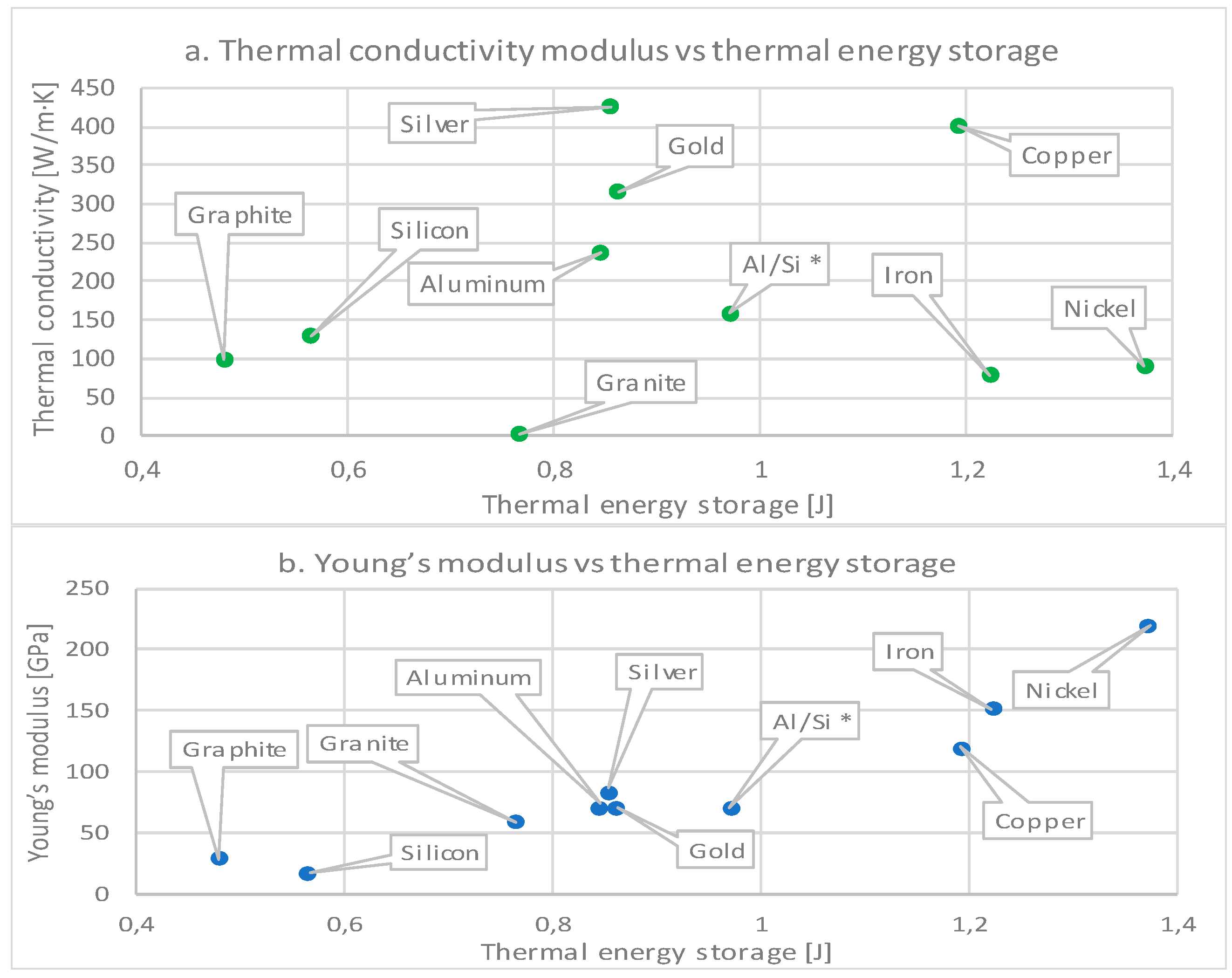
| Material | Thermal Conduc. (W/m·K) | Density (kg/m3) | Specific Heat (J/kg·K) | Thermal Expansion (1/K) | Melting Temperature (°C) [48] | Poisson Ratio [5] | Young’s Modulus (GPa) |
|---|---|---|---|---|---|---|---|
| Nickel | 90.7 | 8900 | 445 | 1.34 × 10−5 | 1453 | 0.31 | 219 |
| Iron | 80.2 | 7860 | 449 | 1.18 × 10−5 | 1535 | 0.27 | 152 |
| Copper | 401 | 8960 | 384 | 1.65 × 10−5 | 1083 | 0.34 | 120 |
| Al/Si (12/88) | 160 | 2700 | 1038 | 2.19 × 10−5 | 830 | 0.33 | 71 |
| Gold | 317 | 19,300 | 129 | 1.42 × 10−5 | 1064 | 0.44 | 70 |
| Silver | 429 | 10,500 | 235 | 1.89 × 10−5 | 962 | 0.37 | 83 |
| Aluminum | 237 | 2700 | 904 | 2.31 × 10−5 | 660 | 0.35 | 70 |
| Granite | 2.9 | 2600 | 850 | 7.00 × 10−5 | 1215–1260 | 0.25 | 60 |
| Silicon Carbide | 450 | 3200 | 1200 | 3.80 × 10−5 | 2730 | 0.18 | 450 |
| Silicon | 130 | 2329 | 700 | 2.60 × 10−5 | 1410 | 0.28 | 17 |
| Graphite | 100 | 1950 | 710 | 7.50 × 10−5 | 3550 | 0.35 | 30 |
| PCM | State | Density (kg/m3) | Thermal Conductivity (W/m·K) | Specific Heat (J/kg·K) | Melting Points (K) | Latent Heat (kJ/kg) |
|---|---|---|---|---|---|---|
| LiNO3 | Solid | 2380 [71] | 0.6 [72] | 1700 [31] | 526 [73] | 373 [73] |
| Liquid | 1780 [31] | 0.7 [72] | 2100 [72] | |||
| NaNO3 | Solid | 2113 [74] | 0.6 [75] | 1655 [74] | 581 [75] | 172 [74] |
| Liquid | 1908 [74] | 0.51 [75] | 1655 [74] | |||
| MgCl2 | Solid | 2230 [75] | 0.6 [75] | 798 [21] | 987 [75] | 454 [75] |
| Liquid | 1675 [75] | 1.2 [75] | 974 [75] | |||
| KNO3/NaNO3 (40/60 wt %) | Solid | 2192 [76] | 0.78 [77] | 1430 [76] | 496 [36] | 105 [36] |
| Liquid | 2096 [76] | 0.45 [77] | 1540 [76] | |||
| NaCl/MgCl2 (57/43 mol %) | Solid | 2072 [21] | 0.5 [78] | 874 [37] | 717 [79] | 292 [37] |
| Liquid | 1750 [80] | 0.5 [78] | 1100 [37] | |||
| LiNO3/KNO3/NaNO3 (30/50/20 wt %) | Solid | 2088 [79] | 0.45 [79] | 1500 [79] | 393 [79] | 155 [81] |
| Liquid | 1720 [82] | 0.45 [81] | 2320 [82] |
| Shell | PCM | Size | Charged Temp. (°C) | Energy Stored (kJ/kg) | Encapsulation Method |
|---|---|---|---|---|---|
| Copper [83] | NaNO3 | 76.2 Diameter × 254 Height | 425 | 211 | Macro-encapsulation |
| Carbon Steel 1018, Stainless steel 304 [84] | NaNO3 | - | 308 | 177 | External coating |
| Ni 200/201 [84] | KOH | - | 280 | 150 | External coating |
| Fe-Ni-Cr-Co and Co-Ni-Cr-W alloys [84] | Zn/Al 95.96/4.00 | - | 381 | 138 | Preformed shell |
| Ti and its alloys [84] | Al/Si/Mg 83.14/11.70/5.16 | - | 555 | 485 | Preformed shell |
| Ti and its alloys [84] | Al/Cu/Mg 70.60/25.46/3.94 | - | 560 | 545 | Preformed shell |
| Stainless steel 304 [85] | Zinc | 25.4 mm Diameter × 50.8 mm Height | 455 | 113 | Macro-encapsulation |
| Stainless steel 304 [85] | Aluminum | 25.4 mm Diameter × 50.8 mm Height | 710 | 398 | Macro-encapsulation |
| Carbon Steel 1018 [85] | NaCl/MgCl2 eutectic | 50.8 mm Diameter × 127 mm Height | 490 | 700 | Macro-encapsulation |
| Stainless steel 304 [85] | MgCl2 | 25.4 mm Diameter × 50.8 mm Height | 745 | 1000 | Macro-encapsulation |
| Stainless steel 304 [85] | NaCl | 25.4 mm Diameter × 50.8 mm Height | 830 | 1200 | Macro-encapsulation |
| Polyurea [86] | Butyl stearate | 20–35 μm Diameter | 29 | 80 | Interfacial poly-condensation |
| Silica gel polymer [86] | Paraffin/HDPE | - | 18.55–22.60 | 24.94–153.46 | In Situ Polymerization |
| PMMA [37] | Docosane | 0.16 μm Diameter | 41 | 54.6 | Emulsion Polymerization |
| PMMA [37] | Paraffin Wax | 0.21 μm Diameter | 49.7 | 106.9 | UV irradiation-initiated polymerization |
| PMMA [37] | Paraffin | 0.2 μm Diameter | 26.24 | 84.0 | Sol-gel |
| PMMA [37] | n-Octadecane | 0.119 μm Diameter | 31.9 | 208.7 | Miniemulsion Polymerization |
| PTFE [87] | NaNO3 | - | 326 | 170 | - |
| PTFE + Nickel coating [87] | NaNO3 | - | 326 | 172 | - |
| PTFE + Nickel coating [87] | KNO3 | - | 350 | 92 | - |
| Material | Strain Amplitude (%) | Stress Amplitude (MPa) | Life Cycles Nf |
|---|---|---|---|
| Fe (Armco) | 0.039% | 181 (20 °C) | >106 |
| 0.120% | 230 (300 °C) | ||
| Ni | 0.081% | 160 (20 °C) | >106 |
| Cu | 0.062% | 77 (20 °C) | >106 |
| 0.034% | 40 (300 °C) | ||
| Al | 0.038% | 27 (20 °C) | >106 |
| AlSi12 casting [89] | n.a. | 100 (20 °C) | >106 |
| 60 (300 °C) | |||
| Al alloys [89]: 319 Al 75S-T6 | n.a. | 38 | >106 |
| 123 | |||
| Steel alloys [90]: 517 340 | n.a. | 420 | >106 |
| 340 | |||
| Carbon steel API 5L [91] | 0.5 | 453 | 1466 |
| 1.0 | 531 | 467 | |
| Ni-based GH4169 superalloy [92] | 0.5 | 930 | 25,956 |
| 1.0 | 1110 | 1516 | |
| Cu alloy (8% at. Al) [93] | 2 | n.a. | 505 |
| 4 | n.a. | 128 | |
| - | 450 | 10,000 | |
| Al alloy 2024-T3 (5% Cu) [94] | 0.6 | 380 (20 °C) | 14,568 |
| 1.0 | 431 (20 °C) | 2614 | |
| 0.6 | 334 (300 °C) | 3719 | |
| 1.0 | 358 (300 °C) | 444 | |
| Al alloy 6061-T6 [95] | 0.5 | n.a. | 28,612 |
| 1.0 | 6855 |
| Material | Cost of Materials ($USD) | Cost of Tanks ($USD) | Sum of Costs ($USD) (Materials + Tanks) |
|---|---|---|---|
| Solar salt a (sensible heating) | $35,817,291.09 | $8,464,500.00 | $44,281,791.09 |
| PCM b with iron shell | $28,947,482.50 | $5,202,570.92 | $34,150,053.43 |
| PCM b with copper shell | $231,541,413.06 | $5,223,805.85 | $236,765,218.90 |
| PCM b with nickel shell | $412,961,521.47 | $5,078,700.13 | $418,040,221.60 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres, G.; Fullenkamp, K.; Montané, M.; Naplocha, K.; Dmitruk, A. Encapsulated Nitrates Phase Change Material Selection for Use as Thermal Storage and Heat Transfer Materials at High Temperature in Concentrated Solar Power Plants. Energies 2017, 10, 1318. https://doi.org/10.3390/en10091318
Cáceres G, Fullenkamp K, Montané M, Naplocha K, Dmitruk A. Encapsulated Nitrates Phase Change Material Selection for Use as Thermal Storage and Heat Transfer Materials at High Temperature in Concentrated Solar Power Plants. Energies. 2017; 10(9):1318. https://doi.org/10.3390/en10091318
Chicago/Turabian StyleCáceres, Gustavo, Karina Fullenkamp, Macarena Montané, Krzysztof Naplocha, and Anna Dmitruk. 2017. "Encapsulated Nitrates Phase Change Material Selection for Use as Thermal Storage and Heat Transfer Materials at High Temperature in Concentrated Solar Power Plants" Energies 10, no. 9: 1318. https://doi.org/10.3390/en10091318
APA StyleCáceres, G., Fullenkamp, K., Montané, M., Naplocha, K., & Dmitruk, A. (2017). Encapsulated Nitrates Phase Change Material Selection for Use as Thermal Storage and Heat Transfer Materials at High Temperature in Concentrated Solar Power Plants. Energies, 10(9), 1318. https://doi.org/10.3390/en10091318




