Exploring PANI-TiN Nanoparticle Coatings in a PEFC Environment: Enhancing Corrosion Resistance and Conductivity of Stainless Steel Bipolar Plates
Abstract
:1. Introduction
2. Experimental
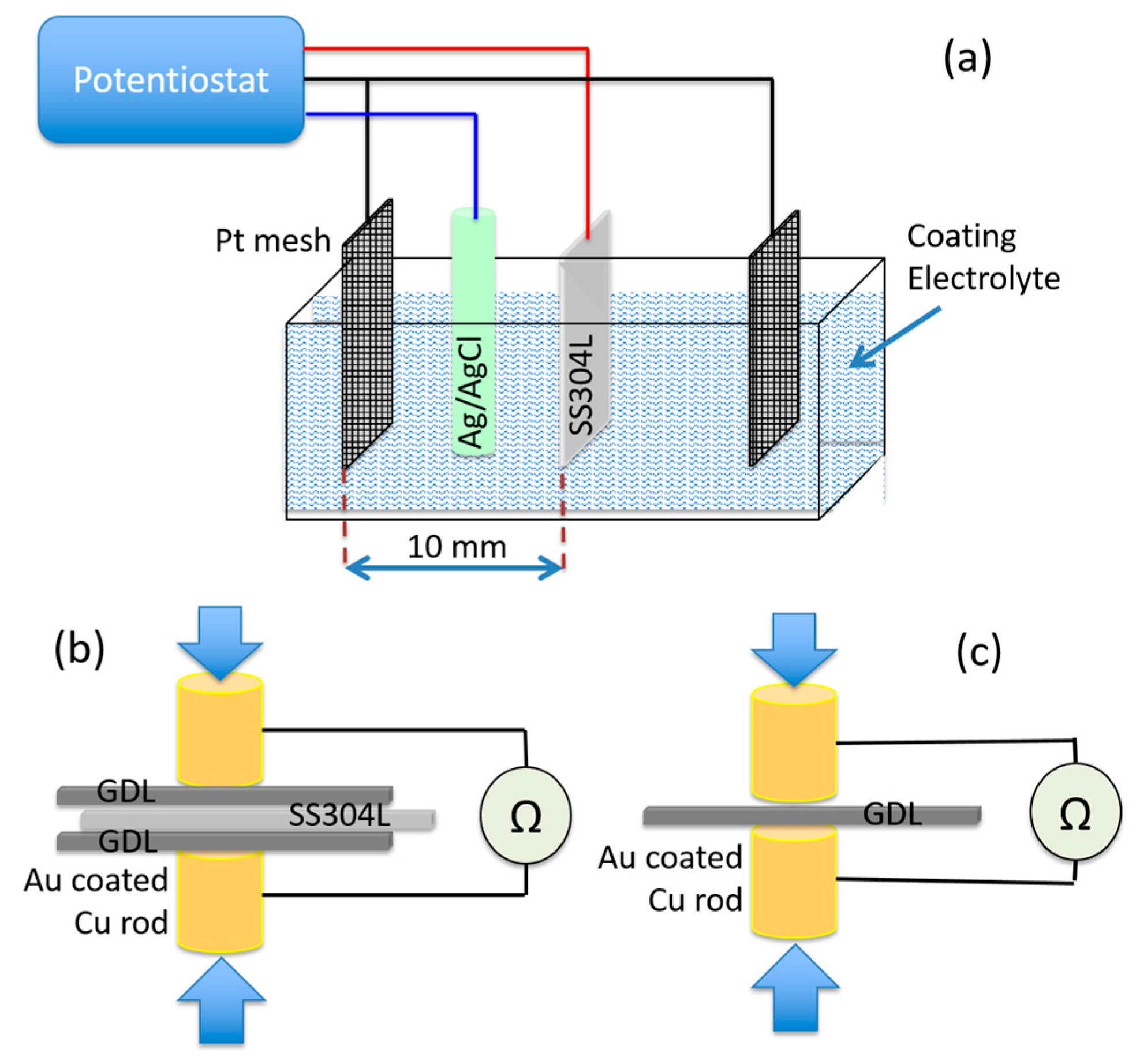
2.1. Preparation and Application of Coating
2.2. Coating Characterisation
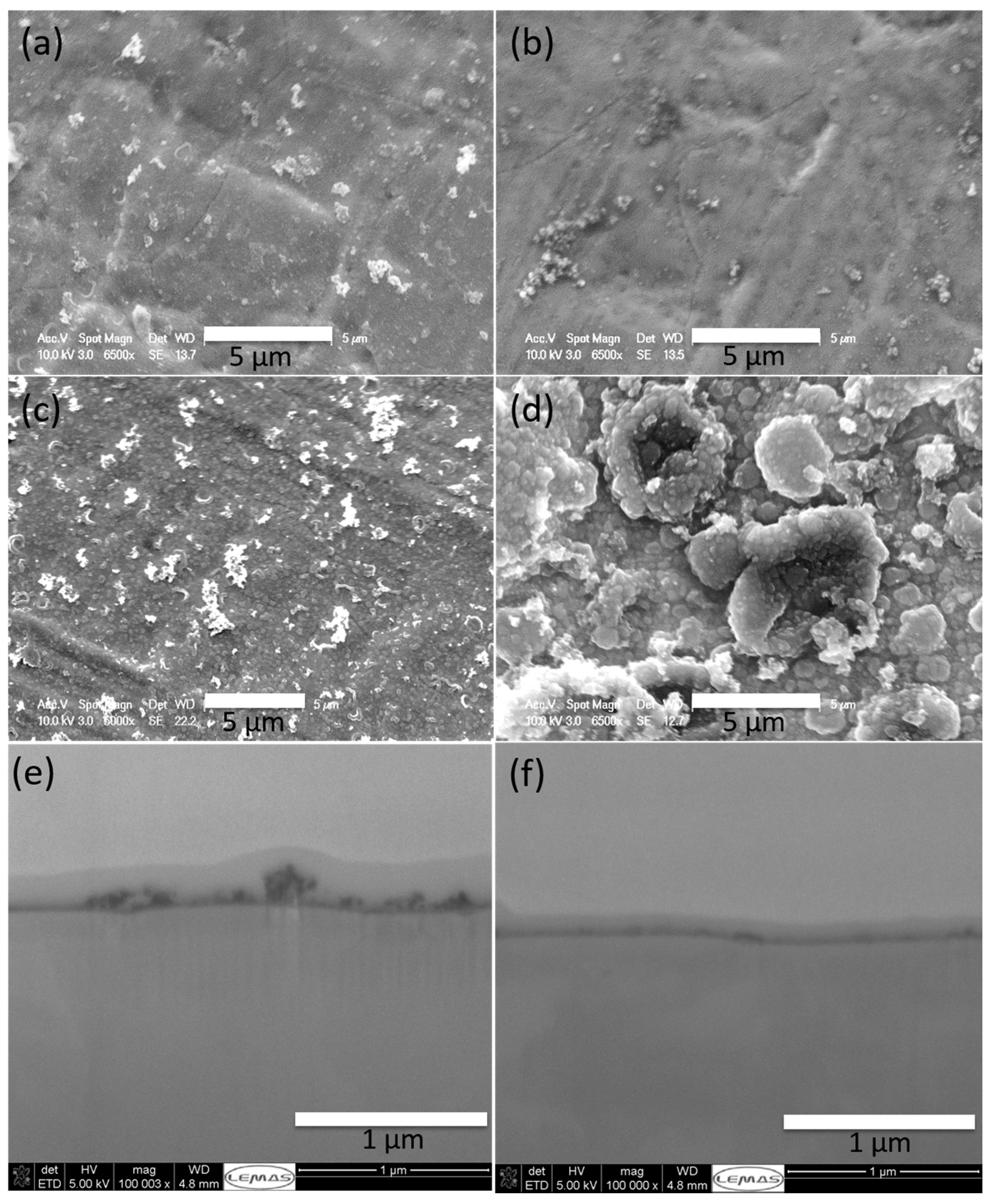
2.2.1. Coating Morphology and Composition
2.2.2. Interfacial Contact Resistance (ICR)
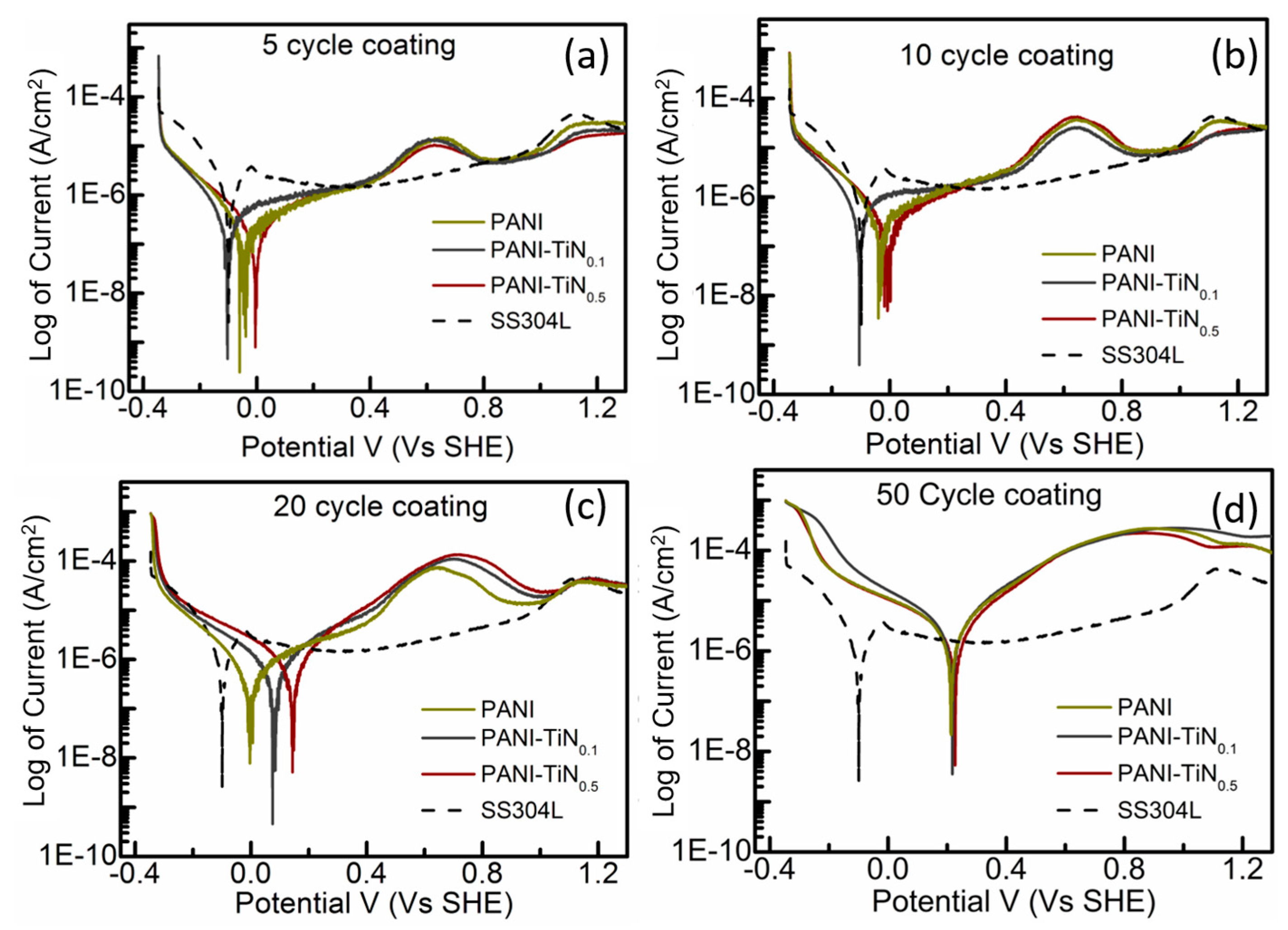
2.2.3. Electrochemical Corrosion
3. Results and Discussion
4. Effect of TiN NP Loading and Coating Thickness
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- André, J.; Antoni, L.; Petit, J.P. Corrosion resistance of stainless steel bipolar plates in a PEFC environment: A comprehensive study. Int. J. Hydrogen Energy 2010, 35, 3684–3697. [Google Scholar] [CrossRef]
- Makkus, R.C.; Janssen, A.H.H.; de Bruijn, F.A.; Mallant, R.K.A.M. Use of Stainless steel of cost competitive bipolar plates in the SPFC. J. Power Sources 2000, 86, 274–282. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhari, T.; Spagnol, P. Bipolar Plates for PEM Fuel Cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Wang, H.; Sweikart, M.A.; Turner, J.A. Stainless steel as bipolar plate material for polymer electrolyte membrane fuel cell. J. Power Sources 2003, 115, 243–251. [Google Scholar] [CrossRef]
- Shanian, A.; Savadogo, O. TOPSIS multipla-criteria decision support analysis for selection of metal bipolar plates for polymer electrolyte fuel cell. J. Power Sources 2006, 159, 1095–1104. [Google Scholar] [CrossRef]
- Silva, R.F.; Franchi, D.; Leone, A.; Pilloni, L.; Masci, A.; Pozio, A. Surface conductivity and stability of metallic bipolar plate materials for polymer electrolyte fuel cells. Electrochim. Acta 2006, 51, 3592–3598. [Google Scholar] [CrossRef]
- Liang, C.-H.; Cao, C.H.; Huang, N.-B. Electrochemical behavior of 304 stainless steel with electrodeposited niobium as PEMFC bipolar plates. Int. J. Miner. Metall. Mater. 2012, 19, 328–332. [Google Scholar] [CrossRef]
- Chung, C.Y.; Chen, S.K.; Chiu, P.J.; Chang, M.H.; Hung, T.T.; Ko, T.H. Carbon film coated 304 stainless steel as PEMFC bipolar plate. J. Power Sources 2008, 176, 276–281. [Google Scholar] [CrossRef]
- Dur, E.; Core, O.N.; Koc, M. Experimental investigations on the corrosion resistance characteristics of coated metallic bipolar plates for PEMFC. Int. J. Hydrogen Energy 2011, 36, 7162–7173. [Google Scholar] [CrossRef]
- Wang, Y.; Northwood, D.O. Effects of O2 and H2 on the corrosion of SS316L metallic bipolar plate materials in simulated anode and cathode environments of PEM fuel cells. Electrochim. Acta 2007, 52, 6793–6798. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Tawfik, H.; Hung, Y.; Mahajan, D. Metal bipolar plates for PEM fuel cells: A review. J. Power Sources 2007, 163, 755–767. [Google Scholar] [CrossRef]
- El-kharouf, A.; Chandan, A.; Hattenberger, M.; Pollet, B.G. Proton exchange membrane fuel cells degradation and testing: Review. J. Energy Inst. 2012, 85, 188–200. [Google Scholar] [CrossRef]
- Pozio, A.; Silva, R.F.; De Francesco, M.; Giorgi, L. Nafion degradation in PEFCs from end plate iron contamination. Electrochim. Acta 2003, 48, 1543–1549. [Google Scholar] [CrossRef]
- Karimi, S.; Fraser, N.; Roberts, B.; Foulkes, F.R. A Review of Metallic Bipolar Plates for Proton Exchange Membrane Fuel Cells: Materials and Fabrication Methods. Adv. Mater. Sci. Eng. 2012, 2012, 828070. [Google Scholar] [CrossRef]
- Lee, S.H.; Kakati, N.; Maiti, J.; Jee, S.H.; Kalita, D.J.; Yoon, Y.S. Corrosion and electrical properties of CrN-, TiN coated 316L stainless steel used as bipolar plates for polymer electrolyte membrane fuel cells. Thin Solid Films 2013, 529, 374–379. [Google Scholar] [CrossRef]
- Nam, N.D.; Jo, D.S.; Kim, J.G.; Yoon, D.H. Transparent amorphous In-Ga-Zn-O thin film as function of various gas flows for TFT applications. Thin Solid Films 2011, 519, 4078–4081. [Google Scholar] [CrossRef]
- Tian, R.; Sun, J. Corrosion resistance and interfacial contact resistance of TiN coated 316L bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 6788–6794. [Google Scholar] [CrossRef]
- Jin, C.K.; Lee, K.H.; Kang, C.G. Performance and characteristics of titanium nitride, chromium nitride multi-coated stainless tell 304 bipolar plates fabricated through rubber forming process. Int. J. Hydrogen Energy 2015, 40, 6681–6688. [Google Scholar] [CrossRef]
- Asri, N.F.; Husaini, T.; Sulong, A.B.; Majlan, E.H.; Ramli, W.; Daud, W. Coating of stainless steel and titanium bipolar plates for anti-corrosion in PEMFC: A review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Omrani, M.; Habibi, M.; Amrollahi, R.; Khosravi, A. Improvement of corrosion and electrical conductivity of 316L stainless steel as bipolar plate by TiN Nanoparticle implantation using plasma focus. Int. J. Hydrogen Energy 2012, 37, 14676–14686. [Google Scholar] [CrossRef]
- Heras, N.D.-L.; Roberts, E.P.L.; Langton, R.; Hodgson, D.R. A Review of Metal Separator plate Materials Suitable for Automotive PEM Fuel Cells. Energy Environ. Sci. 2009, 2, 206–214. [Google Scholar] [CrossRef]
- Bazzaoui, M.; Martins, J.I.; Bazzaoui, E.A.; Albourine, A.; Martins, L. Corrosion protection of stainless steel plates in fuel cell environment by conducting polymers. Mater. Corros. 2014, 65, 67–75. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Makhlouf, A.S.H. Handbook of Smart Coatings for Materials Protection; Woodhead Publishing: Cambridge, UK, 2014; pp. 459–486. ISBN 978-0-85709-680-7. [Google Scholar]
- Ren, Y.J.; Zeng, C.L. Effect of conducting composite polypyrrole/polyanilie coatings on the corrosion resistance of type 304 Stainless steel for bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2008, 182, 524–530. [Google Scholar] [CrossRef]
- Yavuz, A.G.; Gök, A. Preparation of TiO2/PANI composites in presence of surfactants and investigation of electrical properties. Synth. Met. 2007, 157, 235–242. [Google Scholar] [CrossRef]
- Joseph, S.; McClure, J.C.; Chianelli, R.; Pich, P.; Sebastian, P.J. Conducting polymer-coated stainless steel bipolar plates for proton exchange membrane fuel cells (PEMFC). Int. J. Hydrogen Energy 2005, 30, 1339–1344. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lee, Y.-B.; Kim, K.-M.; Jeong, M.-G.; Lim, D.-S. Electrically contuctive polymer composite coating on aluminum for PEM fuel cell bipolar plates. Renew. Energy 2013, 54, 46–50. [Google Scholar] [CrossRef]
- Le, D.P.; Yoo, Y.H.; Kim, J.G.; Cho, S.M.; Son, Y.K. Corrosion characteristics of polyaniline coated 316L stainless steel in sulfuric acid containing fluoride. Corros. Sci. 2009, 51, 330–338. [Google Scholar] [CrossRef]
- Ghani, S.; Sharif, R.; Bashir, S.; Ashraf, A.; Shahzadi, S.; Zaidi, A.A.; Rafique, S.; Zafar, N.; Kamboh, A.H. Dye-sensitized solar cells with high performance electrodeposited gold/aniline composite counter electrodes. Mater. Sci. Semicond. Process. 2015, 31, 588–592. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, L. Novel Polyaniline/Titanium nitride Nanocomposites: Controlable structures and electrical/electrochemical properties. J. Phys. Chem. B 2005, 109, 19732–19740. [Google Scholar] [CrossRef] [PubMed]
- Abaci, S.; Nessark, B. Characterization and corrosion protection properties of composite material (PANI + TiO2) coatings on A304 stainless steel. J. Coat. Technol. Res. 2015, 12, 107–120. [Google Scholar] [CrossRef]
- Zhang, K.; Sharma, S. Site-Selective, Low-Loading, Au Nanoparticle—Polyaniline Hybrid Coatings with Enhanced Corrosion Resistance and Conductivity for Fuel Cells. ACS Sustain. Chem. Eng. 2017, 5, 277–286. [Google Scholar] [CrossRef]
- El-Kharouf, A.; Mason, T.J.; Brett, D.J.L.; Pollet, B.G. Ex-situ characterisation of gas diffusion layers of proton exchange membrane fuel cells. J. Power Sources 2012, 218, 393–404. [Google Scholar] [CrossRef]
- Nam, N.D.; Kim, J.G.; Lee, Y.J.; Son, Y.K. Effect of thermal treatment on the corrosion resistance of polyaniline in H2SO4-HF acid mixture solution. Corros. Sci. 2009, 51, 3007–3013. [Google Scholar] [CrossRef]
- Reda, S.M.; Al-Ghannam, S.M. Synthesis and Electrical Properties of Polyaniline Composite with Silver Nanoparticles. Adv. Mater. Phys. Chem. 2012, 2, 75–81. [Google Scholar] [CrossRef]
- Choi, H.S.; Han, D.H.; Hong, W.H.; Lee, J.J. (titanium, Chromium) nitride coatings for bipolar plate for polymer electrolyte fuel cell. J. Power Sources 2009, 189, 2. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Muthukrishnan, S.; Venkatachari, G.; Trivedi, D.C. Corrosion protection of steel by polyaniline pigmented paint coating. Prog. Org. Coat. 2005, 53, 297–301. [Google Scholar] [CrossRef]
- Yoon, W.; Huang, X.; Fazzino, P.; Reifsnider, K.L.; Akkaoui, M.A. Evaluation of coated metallic bipolar plates for polymer electrolyte fuel cells. J. Power Sources 2008, 179, 265–273. [Google Scholar] [CrossRef]
- André, J.; Antoni, L.; Petit, J.P.; De Vito, E.; Montani, A. Electrical contact resistance between stainlesss steel bipolar plates and carbon felt in PEFC: A comprehensive study. Int. J. Hydrogen Energy 2009, 34, 3125–3133. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lai, J.-J.; Huang, C.-H. Stainless steel bipolar plates. J. Power Sources 2005, 145, 362–368. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Epoxy/polyaniline-ZnO nanorods hybrid nanocomposites coatings: Synthesis, characterisation and corrosion protection performance of conducting paints. Prog. Org. Coat. 2014, 77, 146–159. [Google Scholar] [CrossRef]
- Multi-Year Research, Development and Demostration Plan. Available online: https://energy.gov/sites/prod/files/2016/10/f33/fcto_myrdd_fuel_cells.pdf (accessed on 27 February 2017).
- Lin, M.T.; Wan, C.H.; Wu, W. Enhanced corrosion resistance of ss304 stainless steel and titanium coated with alternate layers of TiN and ZrN in a simulated O2 rich environment of a unitized regenerative fuel cell. Int. Electrochem. Sci. 2014, 9, 7832–7845. [Google Scholar]
- Nam, N.D.; Vaka, M.; Hung, N.T. Corrosion behaviour of TiN, TiAlN and TiAlSiN-coated 316L stainless steel in simulated proton exchange membrane fuel cell environment. J. Power Sources 2014, 268, 240–245. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, L.; Guo, L.; Wang, Z.; Zhao, J.; Tuan, W.H.; Niihara, K. TiN-coated titanium as the bipolar plate for PEMFC by multi-arc ion plating. Int. J. Hydrogen Energy 2011, 36, 9155–9161. [Google Scholar] [CrossRef]


| Coating Composition | Aniline Concentration (M) | TiN Concentration (g L−1) | |
|---|---|---|---|
| Coating Type | |||
| PANI | 0.1 | 0 | |
| PANI-TiN (0.1) | 0.1 | 0.1 | |
| PANI-TiN (0.5) | 0.1 | 0.5 | |
| No. of CV Cycles | 50 Cycles | 20 Cycles | 10 Cycles | 5 Cycles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coating Type | Ecorr V | jcorr μA cm−2 PD | jcorr μA cm−2 PS | Ecorr V | jcorr μA cm−2 PD | jcorr μA cm−2 PS | Ecorr V | jcorr μA cm−2 PD | jcorr μA cm−2 PS | Ecorr V | jcorr μA cm−2 PD | jcorr μA cm−2 PS | |
| PANI | 0.214 | 3.4 | 7.4 | −0.003 | 0.66 | 2.3 | −0.033 | 0.35 | 0.9 | −0.057 | 0.30 | 0.5 | |
| PANI-TiN0.1 | 0.218 | 3.39 | 6.6 | 0.075 | 0.61 | 2.8 | −0.106 | 0.77 | 0.7 | −0.103 | 0.34 | 0.3 | |
| PANI-TiN0.5 | 0.227 | 2.84 | 5.2 | 0.144 | 0.60 | 2.4 | 0.004 | 0.30 | 0.6 | 0.005 | 0.29 | 0.4 | |
| Attribute | ICR (mΩ cm2) | Ecorr (mV Vs SHE) | jcorr (μA cm−2) PD | jcorr (μA cm−2) PS | Ref. | |
|---|---|---|---|---|---|---|
| Sample/Coating Details Coating Method | ||||||
| SS304/TiN (200 nm) DC magnetron sputtering | 10 | 423 | -- | -- | [19] | |
| SS304/ZrN/TiN Cathodic arc evaporation PVD | -- | 279 | 0.68 | -- | [44] | |
| SS316L/TiN (1.5 μm) Cathodic arc plasma | -- | 307 | 0.7 | -- | [45] | |
| SS316L/TiN (3 μm) Multi-arc ion plating | 30 | -56 | >1 | 2.4 | [18] | |
| Ti/TiN (3 μm) Mulit-arc ion plating | 2.4 | 424 | 0.0086 | -- | [46] | |
| SS316L/TiN Cathode arc ion plating | 10 | 264 | 2.5 | 2.7 | [16] | |
| SS304/PANI-TiN (10–200 nm) Electrochem. CV | 32 * | 5–227 | 0.29–2.8 | 0.4–5.2 | This study | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Zhang, K.; Gupta, G.; Santamaria, D.G. Exploring PANI-TiN Nanoparticle Coatings in a PEFC Environment: Enhancing Corrosion Resistance and Conductivity of Stainless Steel Bipolar Plates. Energies 2017, 10, 1152. https://doi.org/10.3390/en10081152
Sharma S, Zhang K, Gupta G, Santamaria DG. Exploring PANI-TiN Nanoparticle Coatings in a PEFC Environment: Enhancing Corrosion Resistance and Conductivity of Stainless Steel Bipolar Plates. Energies. 2017; 10(8):1152. https://doi.org/10.3390/en10081152
Chicago/Turabian StyleSharma, Surbhi, Kun Zhang, Gaurav Gupta, and Daniel G. Santamaria. 2017. "Exploring PANI-TiN Nanoparticle Coatings in a PEFC Environment: Enhancing Corrosion Resistance and Conductivity of Stainless Steel Bipolar Plates" Energies 10, no. 8: 1152. https://doi.org/10.3390/en10081152
APA StyleSharma, S., Zhang, K., Gupta, G., & Santamaria, D. G. (2017). Exploring PANI-TiN Nanoparticle Coatings in a PEFC Environment: Enhancing Corrosion Resistance and Conductivity of Stainless Steel Bipolar Plates. Energies, 10(8), 1152. https://doi.org/10.3390/en10081152






