Molecular Structure and Electronic Properties of Triolein Molecule under an External Electric Field Related to Streamer Initiation and Propagation
Abstract
:1. Introduction
2. Theoretical Method
3. Results and Discussion
3.1. Vibrational Frequencies of the Functional Groups of the Triolein Molecule under No External Electric Field
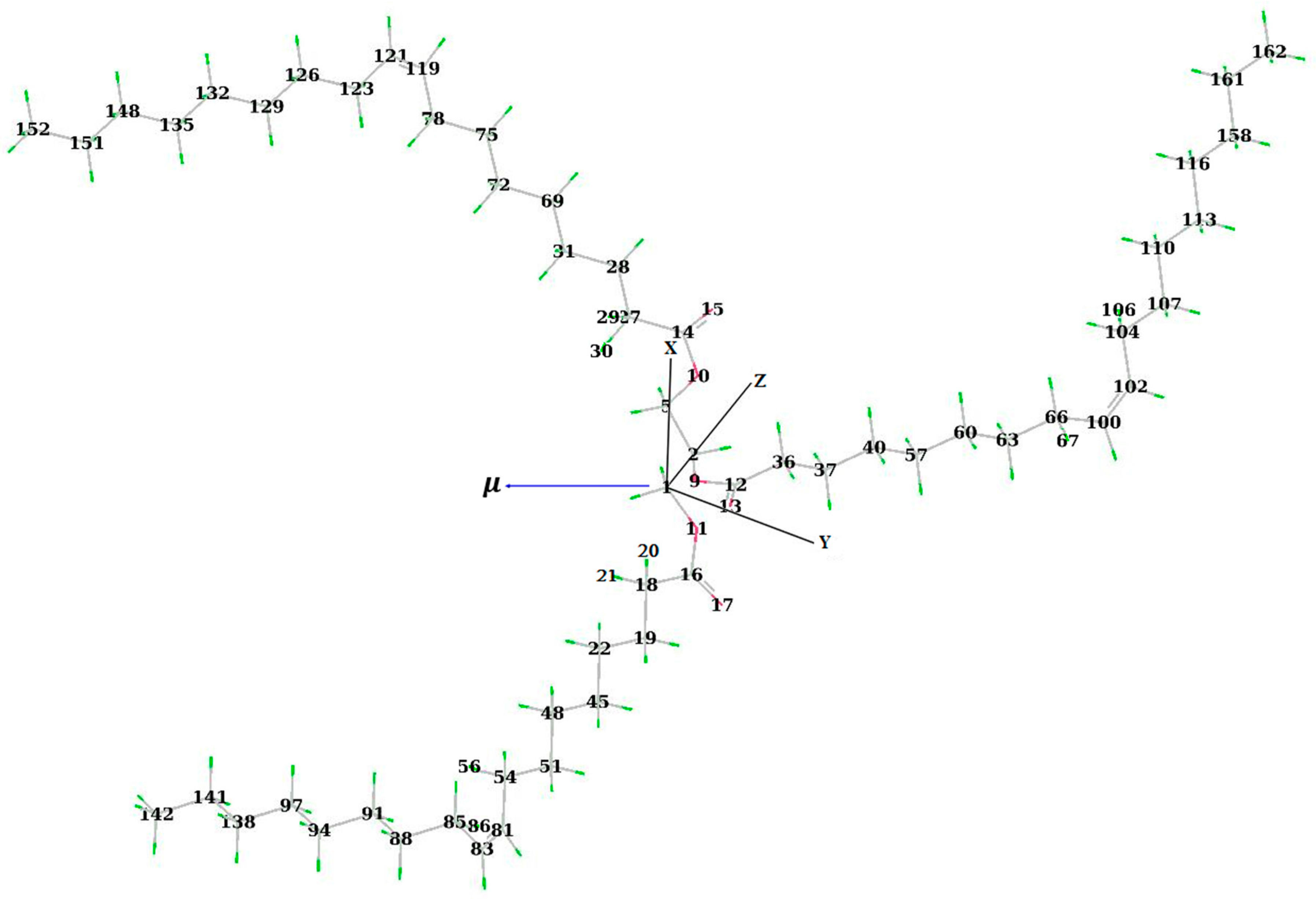
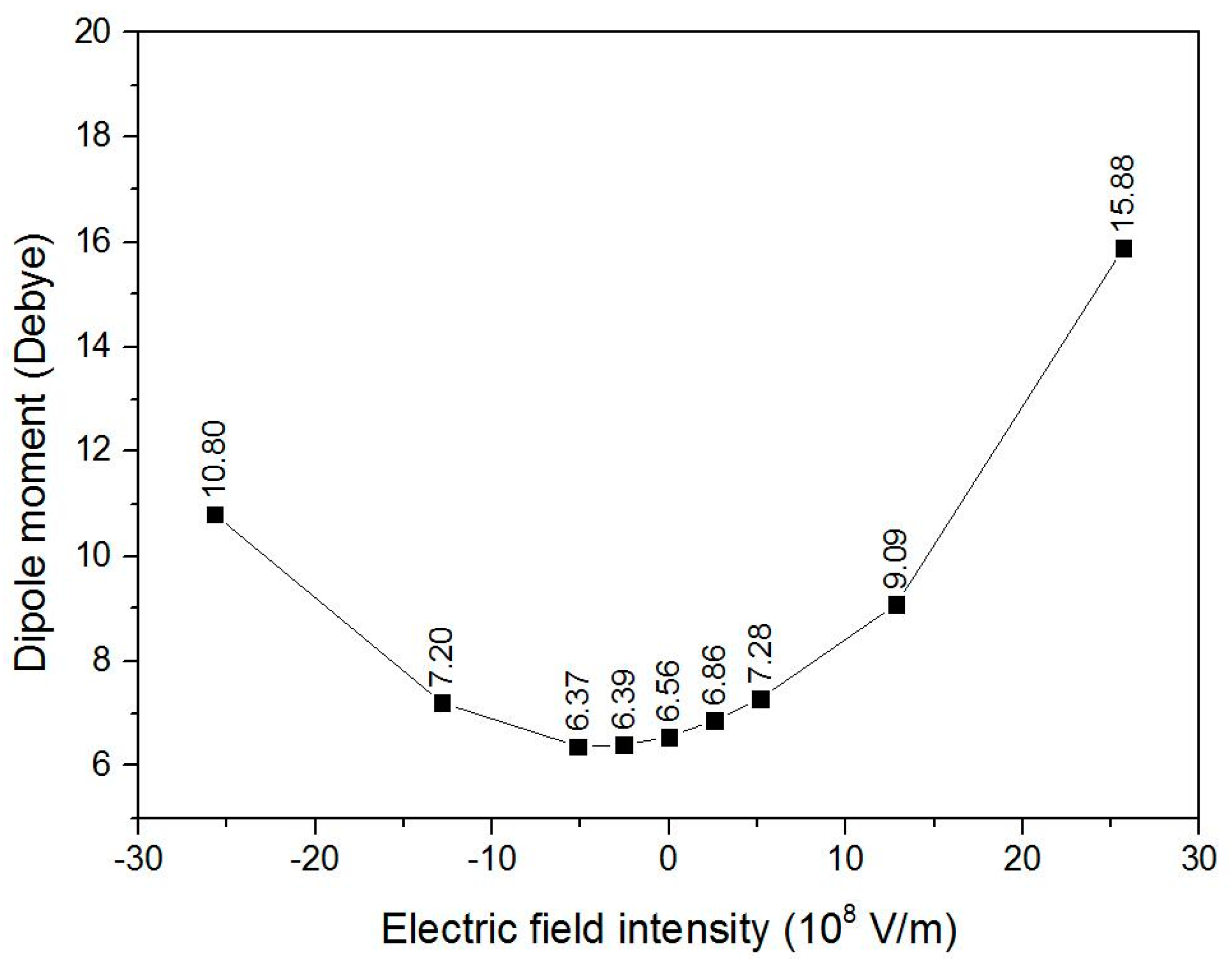
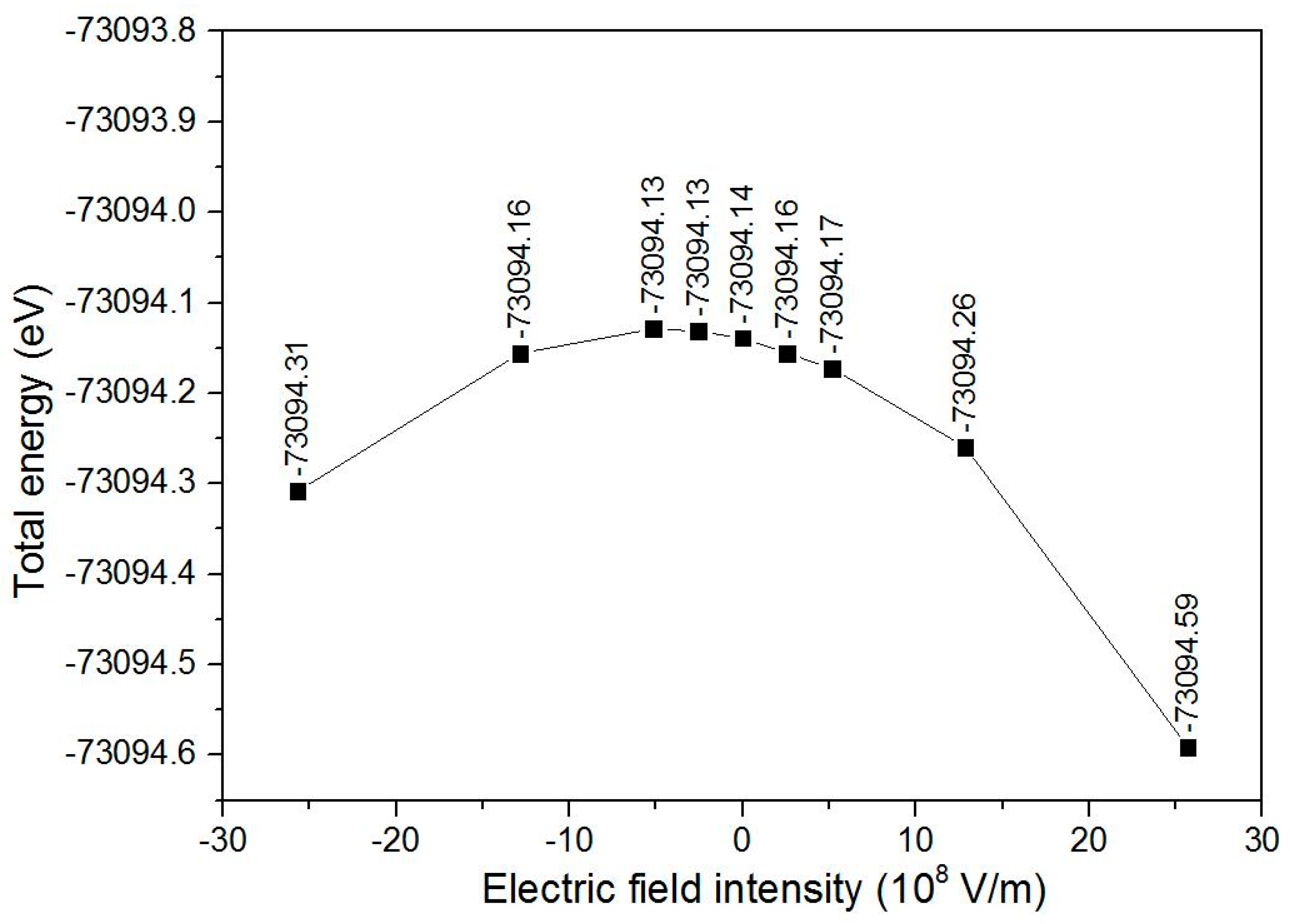
3.2. Bond Lengths, Total Energy, and Dipole Moment of the Triolein Molecule under Different External Electric Fields
3.3. Infrared Spectra of the Triolein Molecule under Different External Electric Fields
3.4. Orbital Energies of the Triolein Molecule under Different External Electric Fields
3.5. Excited States of the Triolein Molecule under Different External Electric Fields
4. Conclusions
- (1)
- The carbon-oxygen bond lengths change obviously with an increasing electric field intensity; however, the carbon-carbon bond lengths change slightly, although changes in the bond lengths are correlated with the direction of the electric field.
- (2)
- The resultant of the permanent dipole moment and the dipole moment induced by the external electric field decreased and then increased when an external electric field was applied along the −Z direction, and increased continuously up to 15.88 Debye under an external electric field intensity of 25.7 × 108 V/m. Accordingly, the total energy increased and then decreased when the external electric field intensity varied from −25.7 × 108 V/m to 25.7 × 108 V/m.
- (3)
- Frequency shifts in IR spectra caused by external electric fields were observed. Frequency shifts were ascribed to changes in the bond lengths. The frequency bands of C-O stretching vibrations, C=O stretching vibrations, and C-H stretching vibrations were extended, while the frequencies of cis C=C rocking vibrations were stable under the external electric fields.
- (4)
- The electronic structure changed obviously under external electric fields. The energy of the HOMO increased with increasing external electric field intensity along both ±Z directions, which represented a corresponding decrease in the IP of the triolein molecule along the ±Z directions. The energy of the LUMO decreased continuously with the external electric field intensity varying from −25.7 × 108 V/m to 25.7 × 108 V/m, which represented a corresponding increase in the EA of the triolein molecule.
- (5)
- The excitation energies tended to decrease with a decreasing external electric field along both the ±Z directions because of the decreasing value of EHLG.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oommen, T.V.; Claiborne, C.C.; Mullen, J.T. Biodegradable electrical insulation fluids. In Proceedings of the Electrical Insulation Conference and Electrical Manufacturing and Coil Winding Conference, Rosemont, IL, USA, 25–25 September 1997; pp. 465–468. [Google Scholar]
- Oommen, T.V. Vegetable oils for liquid-filled transformers. IEEE Electr. Insul. Mag. 2002, 18, 6–11. [Google Scholar] [CrossRef]
- McShane, C.P. Natural and synthetic ester dielectric fluids: Their relative environmental, fire safety, and electrical performance. In Proceedings of the IEEE Industrial and Commercial Power Systems Technical Conference, Sparks, NV, USA, 2–6 May 1999; pp. 8–9. [Google Scholar]
- Svoboda, M.; Trnka, P. Alternative electrical insulating fluids in power transformers. In Proceedings of the 15th International Scientific Conference on Electric Power Engineering, Brno, Czech Republic, 12–14 May 2014; pp. 399–402. [Google Scholar]
- Singh, J.; Sood, Y.R.; Jarial, R.K. Condition monitoring of power transformers—Bibliography survey. IEEE Electr. Insul. Mag. 2008, 24, 11–25. [Google Scholar] [CrossRef]
- Azis, N.; Jasni, J.; Kadir, M.; Mohtar, M.N. Suitability of palm based oil as dielectric insulating fluid in transformers. J. Electr. Eng. Technol. 2014, 9, 662–669. [Google Scholar] [CrossRef]
- Yang, L.J.; Liao, R.J.; Sun, C.X.; Zhu, M.Z. Influence of vegetable oil on the thermal aging of transformer paper and its mechanism. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 692–700. [Google Scholar] [CrossRef]
- Gockenbach, E.; Borsi, H. Natural and synthetic ester liquids as alternative to mineral oil for power transformers. In Proceedings of the Conference on Electrical Insulation and Dielectric Phenomena, Quebec, QC, Canada, 26–29 October 2008; pp. 521–524. [Google Scholar]
- Perrier, C.; Beroual, A. Experimental investigations on insulating liquids for power transformers: Mineral, ester and silicone oils. IEEE Electr. Insul. Mag. 2009, 25, 6–13. [Google Scholar] [CrossRef]
- Dang, V.H.; Beroual, A.; Perrier, C. Comparative study of streamer phenomena in mineral, synthetic and natural ester oils under lightning impulse voltage. In Proceedings of the International Conference on High Voltage Engineering and Application, New Orleans, LA, USA, 11–14 October 2010; pp. 560–563. [Google Scholar]
- Liu, Q.; Wang, Z.D. Streamer characteristic and breakdown in synthetic and natural ester transformer liquids under standard lightning impulse. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 285–294. [Google Scholar] [CrossRef]
- Dang, V.H.; Beroual, A.; Perrier, C. Investigations on streamers phenomena in mineral, synthetic and natural ester oils under lightning impulse voltage. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1521–1527. [Google Scholar] [CrossRef]
- Liu, R.S.; Törnkvist, C. Ester fluids as alternative for mineral oil: The difference in streamer velocity and LI breakdown voltage. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Virginia Beach, VA, USA, 18–21 October 2009; pp. 543–548. [Google Scholar]
- Rapp, K.J.; Corkran, J.; McShane, C.P. Lightning impulse testing of natural ester fluid gaps and insulation interfaces. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1595–1603. [Google Scholar] [CrossRef]
- Denat, A.; Lesaint, O. Breakdown of liquids in long gaps: Influence of distance, impulse shape, liquid nature, and interpretation of measurements. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2581–2591. [Google Scholar] [CrossRef]
- Rapp, K.J.; McShane, C.P.; Vandermaar, J.; Vukovic, D.; Tenbohlen, S. Long gap breakdown of natural ester fluid. In Proceedings of the International Conference on High Voltage Engineering and Application, New Orleans, LA, USA, 11–14 October 2010; pp. 104–107. [Google Scholar]
- Wedin, P. Electrical Breakdown in Dielectric Liquids—A Short Overview. IEEE Electr. Insul. Mag. 2014, 30, 20–25. [Google Scholar] [CrossRef]
- Badent, R.; Hemmer, M.; Konekamp, U.; Julliard, Y.; Schwab, A.J. Streamer inception field strengths in rape-seed oils. In Proceedings of the Conference on Electrical Insulation and Dielectric Phenomena, Victoria, BC, Canada, 15–18 October 2000; pp. 272–275. [Google Scholar]
- Tobazeon, R. Prebreakdown phenomena in dielectric liquids. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 1132–1147. [Google Scholar] [CrossRef]
- Beroual, A.; Zahn, M.; Badent, A.; Kist, K.; Schwab, A.J.; Yamashita, H.; Yamazawa, K.; Danikas, M.; Chadband, W.D.; Torshin, Y. Propagation and structure of streamers in liquid dielectrics. IEEE Electr. Insul. Mag. 1998, 14, 6–17. [Google Scholar] [CrossRef]
- Rozga, P. Streamer propagation in small gaps of synthetic ester and mineral oil under lightning impulse. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1754–2762. [Google Scholar] [CrossRef]
- Lísa, M.; Holčapek, M. Triacylglycerols profiling in plant oils important in food industry, dietetics and cosmetics using high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2008, 1198–1199, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Buckingham, A.D. Direct method of measuring molecular quadrupole moments. J. Chem. Phys. 1959, 30, 1580–1585. [Google Scholar] [CrossRef]
- Gisbergen, S.J.A.; Snijders, J.G.; Baerends, E.J. Implementation of time-dependent density functional response equations. Comput. Phys. Commun. 1999, 118, 119–138. [Google Scholar] [CrossRef]
- Grozema, F.C.; Telesca, R.; Jonkman, H.T.; Siebbeles, L.D.A.; Snijders, J.G. Excited state polarizabilities of conjugated molecules calculated using time dependent density functional theory. J. Chem. Phys. 2001, 15, 10014–10021. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- The National Institute of Standards and Technology (NIST). Computational Chemistry Comparison and Benchmark Data Base. Available online: http://cccbdb.nist.gov/vibscalejust.asp (accessed on 31 October 2016).
- Christy, A.A.; Xu, Z.F.; Harrington, P.D.B. Thermal degradation and isomerisation kinetics of triolein studied by infrared spectrometry and GC–MS combined with chemometrics. Chem. Phys. Lipids 2009, 158, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kos, A.; Tefelski, D.B.; Kosciesza, R.; Rośtocki, A.J.; Roszkiewicz, A.; Ejchart, W.; Jastrzebski, C.; Siegoczyńsk, R.M. Certain physico-chemical properties of triolein and methyl alcohol–triolein mixture under pressure. High Press. Res. 2007, 27, 39–42. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn 3.3.8; Kein Research Center for Natural Sciences: Beijing, China, 2016. [Google Scholar]
- Hebner, R.E.; Kelley, E.F.; Forster, E.O.; FitzPatrick, G.J. Observation of prebreakdown and breakdown phenomena in liquid hydrocarbons II: Non-uniform field conditions. IEEE Trans. Electr. Insul. 1985, 20, 281–292. [Google Scholar] [CrossRef]
- Yamashita, H.; Forster, E.O.; Pompili, M. Streamer formation in perfluoropolyether under impulse conditions. IEEE Trans. Electr. Insul. 1993, 28, 324–329. [Google Scholar] [CrossRef]
- Forster, E.O.; Yamashita, H.; Mazzetti, C.; Pompili, M.; Caroli, L.; Patrissi, S. The effect of the electrode gap on breakdown in liquid dielectrics. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 440–446. [Google Scholar] [CrossRef]
- Nakao, Y.; Yamazaki, T.; Miyagi, K.; Sakai, Y.; Tagashira, H. The effect of molecular structure on prebreakdown phenomena in dielectric liquids under a nonuniform field. Electr. Eng. Jpn. 2002, 139, 1–8. [Google Scholar] [CrossRef]
- Tsuchida, N. The electron affinity and ionization energy of various impurities in silicone oil. IEEE Trans. Dielectr. Electr. Insul. 1993, 28, 243–252. [Google Scholar] [CrossRef]
- Smalø, H.S.; Åstrand, P.O. Calculation of ionization potentials and electron affinities for molecules relevant for streamer initiation and propagation. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 733–741. [Google Scholar] [CrossRef]
- Zener, C. A theory of the electrical breakdown of solid dielectrics. Proc. R. Soc. A 1934, 145, 523–529. [Google Scholar] [CrossRef]
- Sima, W.X.; Jiang, C.L.; Lewin, P.; Yang, Q.; Yuan, T. Modeling of the partial discharge process in a liquid dielectric: Effect of applied voltage, gap distance, and electrode type. Energies 2013, 6, 934–952. [Google Scholar] [CrossRef]
- Jadidian, J.; Zahn, M.; Lavesson, N.; Widlund, O.; Borg, K. Effects of impulse voltage polarity, peak amplitude and rise time on streamer initiated from a needle electrode in transformer oil. IEEE Trans. Plasma Sci. 2012, 40, 909–918. [Google Scholar] [CrossRef]
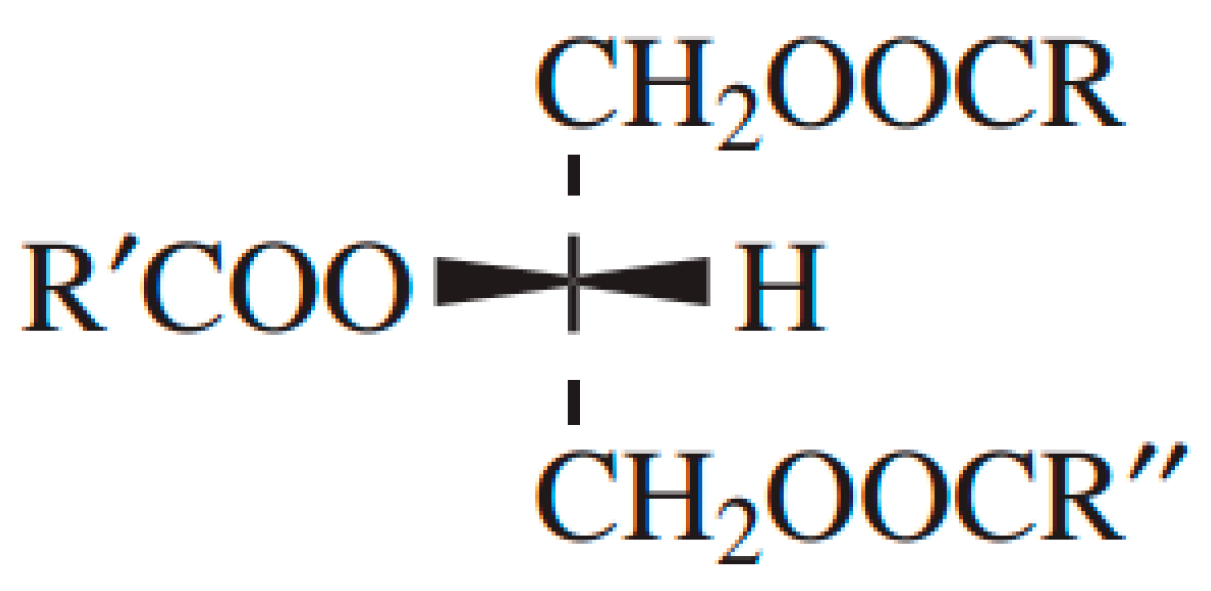






| Method/Scale Factor | Vibration Frequency (cm−1) | |
|---|---|---|
| C=O Stretching | C=C cis Stretching | |
| B3LYP/6-31G*/0.960 1 | 1775.7, 1778.2, 1784.9 | 1673.4, 1673.5, 1673.6 |
| Experimental | 1746 2, 1745 3 | 1653 2,1654 3 |
| E (108 V/m) | R(C1-C2) (Å) | R(C12-O9) (Å) | R(C14-O10) (Å) | R(C14=O15) (Å) | R(C16-O11) (Å) | R(C16=O17) (Å) | R(C100=C102) (Å) |
|---|---|---|---|---|---|---|---|
| −25.7 | 1.5299 | 1.3877 | 1.3806 | 1.2012 | 1.3715 | 1.2081 | 1.3393 |
| −12.85 | 1.5297 | 1.3843 | 1.3759 | 1.203 | 1.3715 | 1.2064 | 1.3391 |
| −5.14 | 1.5295 | 1.382 | 1.3733 | 1.2042 | 1.3754 | 1.2056 | 1.3391 |
| −2.57 | 1.5295 | 1.3812 | 1.3724 | 1.2046 | 1.3761 | 1.2053 | 1.3391 |
| 0 | 1.5295 | 1.3804 | 1.3715 | 1.205 | 1.3767 | 1.2051 | 1.3391 |
| 2.57 | 1.5295 | 1.3796 | 1.3706 | 1.2054 | 1.3773 | 1.2049 | 1.3391 |
| 5.14 | 1.5295 | 1.3787 | 1.3698 | 1.2058 | 1.3779 | 1.2047 | 1.3391 |
| 12.85 | 1.5295 | 1.376 | 1.3672 | 1.2071 | 1.3798 | 1.2042 | 1.3392 |
| 25.7 | 1.529 | 1.3709 | 1.3603 | 1.2101 | 1.3846 | 1.2028 | 1.3393 |
| E (108 V/m) | Main Composition of the HOMO and LUMO (%) | |
|---|---|---|
| −25.7 | HOMO | C100:37.00, C102:30.88, C107:4.86, C104:3.49, C110:2.62, C113:2.61, C116:2.55 |
| LUMO | C16:52.92, O17:26.39, O11:6.80, H21:5.86, H20:3.00, C18:2.23 | |
| −12.85 | HOMO | C100:40.72, C102:37.70, C107:3.84, C63:3.06, C106:2.63, C104:2.74, H67:2.35 |
| LUMO | C16:51.03, O17:25.85, H21:5.29, H20:3.49, C18:2.17, C14:1.10 | |
| −5.14 | HOMO | C100:40.20, C102:39.20, C107:3.59, C63:3.23, H106:2.53, H67:2.49, C104:2.43 |
| LUMO | C14:51.68, O15:26.64, O10:6.35, H30:4.83, H29:3.32, C27:2.20 | |
| −2.57 | HOMO | C100:39.95, C102:39.35, C107:3.51, C63:3.32, C106:2.51, H67:2.50, C104:2.35 |
| LUMO | C14:52.29, O15:26.85, O10:6.49, H30:4.86, H29:3.36, C27:2.22 | |
| 0 | HOMO | C100:39.67, C102:39.65, C107:3.44, C63:3.40, H67:2.52, C106:2.50, C104:2.27 |
| LUMO | C14:52.51, O15:26.87, O10:6.58, H30:4.86, H29:3.38, C27:2.22 | |
| 2.57 | HOMO | C81:39.78, C83:39.77, C88:3.39, C51:3.36, H56:2.52, H86:2.52, C85:2.25 |
| LUMO | C14:52.65, O15:26.85, O10:6.66, H30:4.85, H29:3.39, C27:2.22 | |
| 5.14 | HOMO | C83:39.94, C81:39.80, C88:3.37, C51:3.36, H56:2.51, H86:2.49, C54:2.23 |
| LUMO | C14:52.75, O15:26.80, O10:6.74, H30:4.85, H29:3.40, C27:2.21 | |
| 12.85 | HOMO | C83:40.38, C81:39.89, C51:3.36, C88:3.32, H56:2.45, H86:2.44, C54:2.19 |
| LUMO | C14:53.01, O15:26.64, O10:6.96, H30:4.71, H29:3.50, C27:2.19 | |
| 25.7 | HOMO | C81:40.97, C83:35.21, C88:4.33, C85:3.14, H86:2.59, H56:2.26, C91:1.72 |
| LUMO | C14:53.32, O15:26.26, O10:7.53, H30:4.76, H29:3.51, C27:2.21 |
| E (108 V/m) | n = 1 | n = 2 | n = 3 | n = 4 | n = 5 | n = 6 | n = 7 | n = 8 | |
|---|---|---|---|---|---|---|---|---|---|
| −25.7 | Eex (eV) | 5.68 | 5.71 | 5.72 | 5.92 | 5.96 | 6.05 | 6.11 | 6.12 |
| λ (nm) | 218.33 | 217.28 | 216.94 | 209.43 | 208.05 | 205.10 | 202.88 | 202.68 | |
| f | 0.0005 | 0.0002 | 0.0006 | 0 | 0 | 0 | 0.0001 | 0 | |
| −12.85 | Eex (eV) | 5.70 | 5.71 | 5.73 | 5.96 | 6.02 | 6.08 | 6.19 | 6.23 |
| λ (nm) | 217.45 | 217.21 | 216.23 | 208.17 | 206.05 | 203.85 | 200.27 | 199.14 | |
| f | 0.0005 | 0.0006 | 0.0003 | 0 | 0 | 0 | 0 | 0 | |
| −5.14 | Eex (eV) | 5.70 | 5.71 | 5.74 | 5.98 | 6.05 | 6.10 | 6.23 | 6.29 |
| λ (nm) | 217.44 | 217.2 | 216.14 | 207.44 | 204.8 | 203.11 | 199.16 | 197.48 | |
| f | 0.0005 | 0.0006 | 0.0003 | 0 | 0 | 0 | 0 | 0 | |
| −2.57 | Eex (eV) | 5.70 | 5.71 | 5.74 | 5.98 | 6.06 | 6.11 | 6.23 | 6.29 |
| λ (nm) | 217.54 | 217.17 | 216.16 | 207.23 | 204.46 | 202.89 | 198.9 | 197.12 | |
| f | 0.0005 | 0.0006 | 0.0004 | 0 | 0 | 0 | 0 | 0 | |
| 0 | Eex (eV) | 5.70 | 5.71 | 5.73 | 5.99 | 6.08 | 6.12 | 6.24 | 6.30 |
| λ (nm) | 217.64 | 217.17 | 216.2 | 207.02 | 204.08 | 202.68 | 198.65 | 196.83 | |
| f | 0.0005 | 0.0006 | 0.0004 | 0 | 0 | 0 | 0 | 0 | |
| 2.57 | Eex (eV) | 5.69 | 5.71 | 5.73 | 6.00 | 6.09 | 6.12 | 6.25 | 6.30 |
| λ (nm) | 217.75 | 217.2 | 216.25 | 206.79 | 203.69 | 202.45 | 198.41 | 196.68 | |
| f | 0.0005 | 0.0006 | 0.0004 | 0 | 0 | 0 | 0 | 0 | |
| 5.14 | Eex (eV) | 5.69 | 5.71 | 5.73 | 6.00 | 6.10 | 6.13 | 6.26 | 6.30 |
| λ (nm) | 217.86 | 217.26 | 216.32 | 206.56 | 203.29 | 202.21 | 198.19 | 196.78 | |
| f | 0.0005 | 0.0007 | 0.0004 | 0 | 0 | 0 | 0 | 0 | |
| 12.85 | Eex (eV) | 5.68 | 5.70 | 5.73 | 6.03 | 6.14 | 6.16 | 6.26 | 6.27 |
| λ (nm) | 218.16 | 217.56 | 216.54 | 205.78 | 202.01 | 201.39 | 198.06 | 197.71 | |
| f | 0.0005 | 0.0007 | 0.0004 | 0 | 0 | 0 | 0 | 0 | |
| 25.7 | Eex (eV) | 5.67 | 5.70 | 5.72 | 5.92 | 6.09 | 6.14 | 6.23 | 6.32 |
| λ (nm) | 218.85 | 217.52 | 216.74 | 209.38 | 203.67 | 201.86 | 199.16 | 196.06 | |
| f | 0.0005 | 0.0006 | 0.0003 | 0 | 0 | 0 | 0 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, F.; Li, J.; Huang, Z.; Liang, S.; Zhou, J. Molecular Structure and Electronic Properties of Triolein Molecule under an External Electric Field Related to Streamer Initiation and Propagation. Energies 2017, 10, 510. https://doi.org/10.3390/en10040510
Wang Y, Wang F, Li J, Huang Z, Liang S, Zhou J. Molecular Structure and Electronic Properties of Triolein Molecule under an External Electric Field Related to Streamer Initiation and Propagation. Energies. 2017; 10(4):510. https://doi.org/10.3390/en10040510
Chicago/Turabian StyleWang, Yachao, Feipeng Wang, Jian Li, Zhengyong Huang, Suning Liang, and Jinghan Zhou. 2017. "Molecular Structure and Electronic Properties of Triolein Molecule under an External Electric Field Related to Streamer Initiation and Propagation" Energies 10, no. 4: 510. https://doi.org/10.3390/en10040510






