Reduction of Furfural to Furfuryl Alcohol in Liquid Phase over a Biochar-Supported Platinum Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Support Synthesis and Functionalization
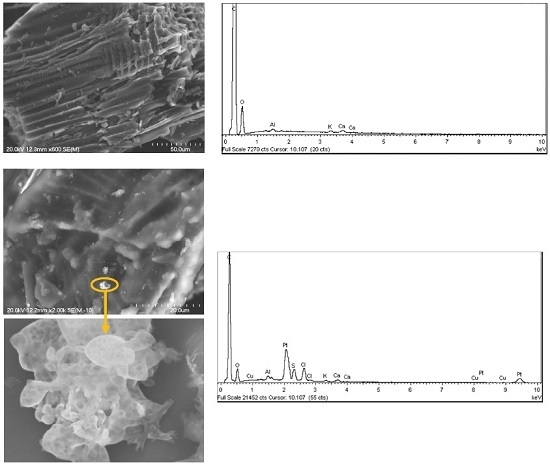
2.2. Catalyst Characterization
2.3. Catalyst Test with Pt/BC
2.4. Catalyst Reactivation Test
3. Experimental
3.1. Support Preparation
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Test
3.5. Catalyst Regeneration Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, R.; Lavoie, J.M. From first- to third-generation biofuels: Challenges of producing a commodity from a biomass of increasing complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Sánchez, O.J.; Montoya, S. Production of Bioethanol from Biomass: An Overview. In Biofuel Technologies; Springer: Berlin/Heidelberg, Germany, 2013; pp. 397–441. [Google Scholar]
- Su, R.; Ma, Y.; Qi, W.; Zhang, M.; Wang, F.; Du, R.; Yang, J.; Zhang, M.; He, Z. Ethanol Production from High-Solid SSCF of Alkaline-Pretreated Corncob Using Recombinant Zymomonas mobilis CP4. BioEnergy Res. 2013, 6, 292–299. [Google Scholar] [CrossRef]
- Tran, A.; Chambers, R.P. Red oak wood derived inhibitors in the ethanol fermentation of xylose by Pichia stipitis CBS 5776. Biotechnol. Lett. 1985, 7, 841–845. [Google Scholar] [CrossRef]
- Fuente-Hernandez, A.; Corcos, P.O.; Beauchet, R.; Lavoie, J.M. Biofuels and co-products out of hemicelluloses. In Liquid, Gaseous and Solid Biofuels-Conversion Techniques; Fang, Z., Ed.; InTech: Rijeka, Croatia, 2013; p. 346. [Google Scholar]
- Yang, W.; Li, P.; Bo, D.; Chang, H.; Wang, X.; Zhu, T. Optimization of furfural production from D-xylose with formic acid as catalyst in a reactive extraction system. Bioresour. Technol. 2013, 133, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, E.; Gallo, J.; Alonso, D.; Wettstein, S.; Lim, W.; Dumesic, J.A. Conversion of Hemicellulose into Furfural Using Solid Acid Catalysts in γ-Valerolactone. Angew. Chem. Int. Ed. 2013, 52, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- O’Driscoll, A.; Leahy, J.J.; Curtin, T. The influence of metal selection on catalyst activity for the liquid phase hydrogenation of furfural to furfuryl alcohol. Catal. Today 2015, 279, 194–201. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Durán-Martín, D.; Moreno-Tost, R.; Santamaría-González, J.; Mérida-Robles, J.; Mariscal, R.; Maireles-Torres, P. Gas-phase hydrogenation of furfural to furfuryl alcohol over Cu/ZnO catalysts. J. Catal. 2016, 336, 107–115. [Google Scholar] [CrossRef]
- Li, M.; Hao, Y.; Cárdenas-Lizana, F.; Keane, M.A. Selective production of furfuryl alcohol via gas phase hydrogenation of furfural over Au/Al2O3. Catal. Commun. 2015, 69, 119–122. [Google Scholar] [CrossRef]
- Wu, J.; Shen, Y.; Liu, C.; Wang, H.; Geng, C.; Zhang, Z. Vapor phase hydrogenation of furfural to furfuryl alcohol over environmentally friendly Cu–Ca/SiO2 catalyst. Catal. Commun. 2005, 6, 633–637. [Google Scholar] [CrossRef]
- Vaidya, P.; MahajaniInd, V. Kinetics of Liquid-Phase Hydrogenation of Furfuraldehyde to Furfuryl Alcohol over a Pt/C Catalyst. Ind. Eng. Chem. Res. 2003, 42, 3881–3885. [Google Scholar] [CrossRef]
- Sitthisa, S.; Pham, T.; Prasomsri, T.; Sooknoi, T.; Mallinson, R.; Resasco, D. Conversion of furfural and 2-methylpentanal on Pd/SiO2 and Pd–Cu/SiO2 catalysts. J. Catal. 2011, 280, 17–27. [Google Scholar] [CrossRef]
- Kijenski, J.; Winiarek, P. Selective hydrogenation of α,β-unsaturated aldehydes over Pt catalysts deposited on monolayer supports. Appl. Catal. A Gen. 2000, 193, L1–L4. [Google Scholar] [CrossRef]
- Nagaraja, B.; Siva Kumar, V.; Shasikala, V.; Padmasri, A.; Sreedhar, B.; Raju, B.; Rama Rao, K. A highly efficient Cu/MgO catalyst for vapour phase hydrogenation of furfural to furfuryl alcohol. Catal. Commun. 2003, 4, 287–293. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, Q.; Zhang, A.; Wu, M. Synthesis of water-soluble monotosylated ethylenediamines and their application in ruthenium and iridium-catalyzed transfer hydrogenation of aldehydes. Appl. Organomet. Chem. 2011, 25, 856–861. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Production of 1,5-pentanediol from biomass via furfural and tetrahydrofurfuryl alcohol. Catal. Today 2012, 195, 136–143. [Google Scholar] [CrossRef]
- Inada, K.; Shibagaki, M.; Nakanishi, Y.; Matsushita, H. The catalytic reduction of aldehydes and ketones with 2-propanol over silica-supported zirconium catalyst. ChemInform 1994, 25. [Google Scholar] [CrossRef]
- Kijenski, J.; Winiarek, P.; Paryjczak, T.; Lewicki, A.; Mikołajska, A. Platinum deposited on monolayer supports in selective hydrogenation of furfural to furfuryl alcohol. Appl. Catal. A Gen. 2002, 233, 171–182. [Google Scholar] [CrossRef]
- Nagaraja, B.; Padmasri, A.; David Raju, B.; Rama Rao, K. Vapor phase selective hydrogenation of furfural to furfuryl alcohol over Cu–MgO coprecipitated catalysts. J. Mol. Catal. A Chem. 2007, 265, 90–97. [Google Scholar] [CrossRef]
- Li, H.; Luo, H.; Zhuang, L.; Dai, W.; Qiao, M. Liquid phase hydrogenation of furfural to furfuryl alcohol over the Fe-promoted Ni-B amorphous alloy catalysts. J. Mol. Catal. A Chem. 2003, 203, 267–275. [Google Scholar] [CrossRef]
- Sitthisa, S.; An, W.; Resasco, D. Selective conversion of furfural to methylfuran over silica-supported Ni-Fe bimetallic catalysts. J. Catal. 2011, 284, 90–101. [Google Scholar] [CrossRef]
- Baijuna, L.; Lianhaia, L.; Bingchuna, W.; Tianxia, C.; Iwatani, K. Liquid phase selective hydrogenation of furfural on Raney nickel modified by impregnation of salts of heteropolyacids. Appl. Catal. A Gen. 1998, 171, 117–122. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Hájek, P.; Salmi, J.; Murzin, T.; Yu, D. Chemoselective hydrogenation of carbonyl compounds over heterogeneous catalysts. Appl. Catal. A Gen. 2005, 292, 1–49. [Google Scholar] [CrossRef]
- Dehkhoda, A.; West, A.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Von Arx, M.; Mallat, T.; Baiker, A. Unprecedented selectivity behaviour in the hydrogenation of an α,β-unsaturated ketone: Hydrogenation of ketoisophorone over alumina-supported Pt and Pd. J. Mol. Catal. A Chem. 1999, 148, 275–283. [Google Scholar] [CrossRef]





| Entry | Catalyst (mmol) | P (psi) | T (°C) | t (h) | Conversion (%) | FA Yield (%) | TOF (s−1) | Selectivity (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | THFA | MF | F | ||||||||
| 1 | 0.025 | 500 | 210 | 2 | 20.1 | 6.9 | 4.0×10−2 | 34.2 | - | 6.4 | 40.1 |
| 2 | 0.025 | 500 | 250 | 2 | 18.4 | 3.9 | 3.7×10−2 | 21.2 | - | 12.6 | 63.4 |
| 3 | 0.025 | 1000 | 210 | 2 | 35.6 | 25.7 | 7.1×10−2 | 72.2 | - | 6.6 | 20.1 |
| 4 | 0.025 | 1000 | 250 | 2 | 36.4 | 17.0 | 7.3×10−2 | 46.7 | 0.4 | 14.7 | 33.8 |
| 5 | 0.058 | 1000 | 250 | 2 | 52.0 | 13.8 | 4.5×10−2 | 26.6 | 0.1 | 13.0 | 58.8 |
| 6 | 0.058 | 1000 | 250 | 1 | 30.1 | 11.4 | 5.2×10−2 | 38.0 | - | 14.5 | 46.2 |
| 7 | 0.025 | 1000 | 250 | 3 | 40.8 | 20.2 | 5.4×10−2 | 49.6 | 0.2 | 19.1 | 29.8 |
| 8 | 0.025 | 1500 | 170 | 2 | 19.4 | 16.1 | 3.9×10−2 | 83.2 | 0.5 | 5.1 | 9.0 |
| 9 | 0.025 | 1500 | 210 | 2 | 60.8 | 48.2 | 1.2×10−2 | 79.2 | 0.4 | 8.5 | 11.0 |
| 10 | 0.025 | 1500 | 210 | 4 | 66.9 | 55.4 | 6.7×10−2 | 82.8 | 1.1 | 0.9 | 13.4 |
| 11 | 0.025 | 1500 | 210 | 6 | 69.4 | 49.3 | 4.6×10−2 | 71.1 | 0.7 | 12.8 | 14.7 |
| 12 | 0.025 | 1500 | 250 | 2 | 33.4 | 27.1 | 6.7×10−2 | 81.1 | 1.0 | 8.8 | 7.7 |
| 13 | 0.025 | 1500 | 320 | 2 | 70.0 | 11.8 | 1.4×10−2 | 16.8 | 0.6 | 35.2 | 43.2 |
| 14 | 0.025 | 1500 | 300 | 3 | 59.9 | 10.1 | 8.0×10−2 | 16.9 | 1.2 | 41.6 | 37.3 |
| Entry | Catalyst (mmol) | t (h) | Conversion (%) | FA Yield (%) | TOF (s−1) | Selectivity (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| FA | THFA | MF | F | ||||||
| 9 | 0.025 (3%) | 2 | 60.8 | 48.2 | 1.2 × 10−2 | 79.2 | 0.4 | 8.5 | 11.0 |
| 10 | 4 | 66.9 | 55.4 | 6.7 × 10−2 | 82.8 | 1.1 | 0.9 | 13.4 | |
| 15 | 0.044 (5%) | 2 | 40.5 | 31.3 | 4.6 × 10−2 | 77.3 | 0.5 | 4.3 | 12.0 |
| 16 | 4 | 45.3 | 32.4 | 2.5 × 10−2 | 71.6 | 0.4 | 6.2 | 16.6 | |
| Entry | Solvent | T (°C) | Conversion (%) | FA Yield (%) | TOF (s−1) | Selectivity (%) | |
|---|---|---|---|---|---|---|---|
| FA | MF | ||||||
| 12 | Toluene | 250 | 33.4 | 27.1 | 6.7 × 10−2 | 81.1 | 8.8 |
| 9 | 210 | 60.8 | 48.1 | 1.2 × 10−1 | 79.2 | 8.5 | |
| 17 | Isopropanol | 250 | 37.5 | 21.1 | 7.5 × 10−2 | 56.4 | 23.4 |
| 18 | 210 | 42.9 | 34.9 | 8.6 × 10−2 | 81.3 | 9.0 | |
| 19 | Isobutanol | 250 | 57.1 | 50.6 | 1.1 × 10−1 | 88.6 | - |
| 20 | 210 | 82.8 | 49.6 | 1.7 × 10−1 | 59.9 | - | |
| 21 | Hexane | 250 | 66.4 | 16.1 | 1.3 × 10−1 | 24.2 | - |
| 22 | 210 | 52.3 | 41.9 | 1.0 × 10−1 | 80.2 | - | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuente-Hernández, A.; Lee, R.; Béland, N.; Zamboni, I.; Lavoie, J.-M. Reduction of Furfural to Furfuryl Alcohol in Liquid Phase over a Biochar-Supported Platinum Catalyst. Energies 2017, 10, 286. https://doi.org/10.3390/en10030286
Fuente-Hernández A, Lee R, Béland N, Zamboni I, Lavoie J-M. Reduction of Furfural to Furfuryl Alcohol in Liquid Phase over a Biochar-Supported Platinum Catalyst. Energies. 2017; 10(3):286. https://doi.org/10.3390/en10030286
Chicago/Turabian StyleFuente-Hernández, Ariadna, Roland Lee, Nicolas Béland, Ingrid Zamboni, and Jean-Michel Lavoie. 2017. "Reduction of Furfural to Furfuryl Alcohol in Liquid Phase over a Biochar-Supported Platinum Catalyst" Energies 10, no. 3: 286. https://doi.org/10.3390/en10030286
APA StyleFuente-Hernández, A., Lee, R., Béland, N., Zamboni, I., & Lavoie, J.-M. (2017). Reduction of Furfural to Furfuryl Alcohol in Liquid Phase over a Biochar-Supported Platinum Catalyst. Energies, 10(3), 286. https://doi.org/10.3390/en10030286