Effect of Coal Grain Size on Sorption Capacity with Respect to Propylene and Acetylene
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
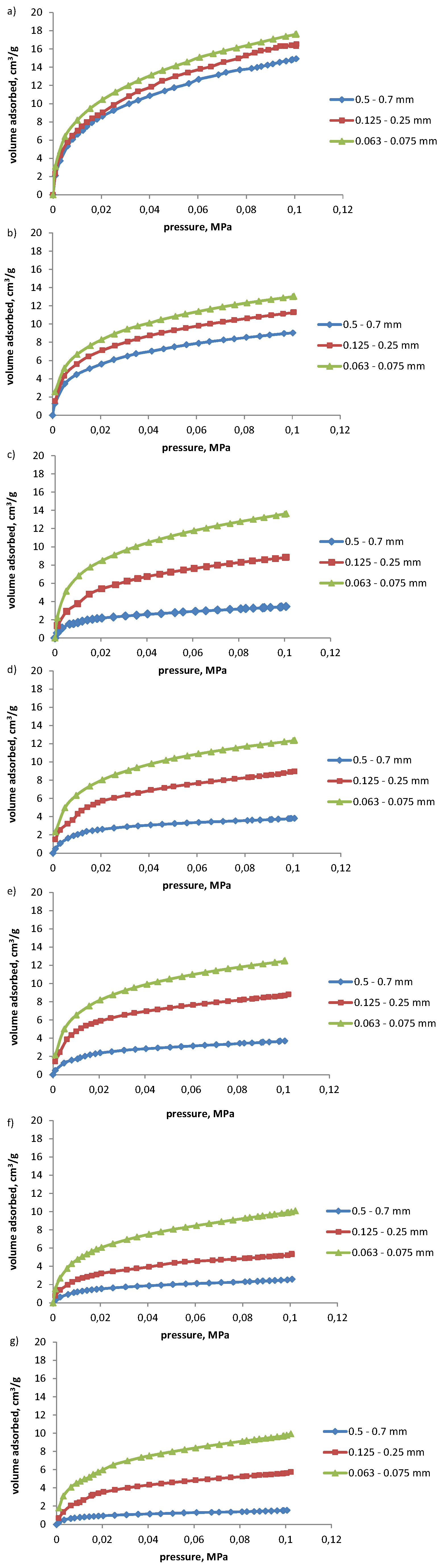
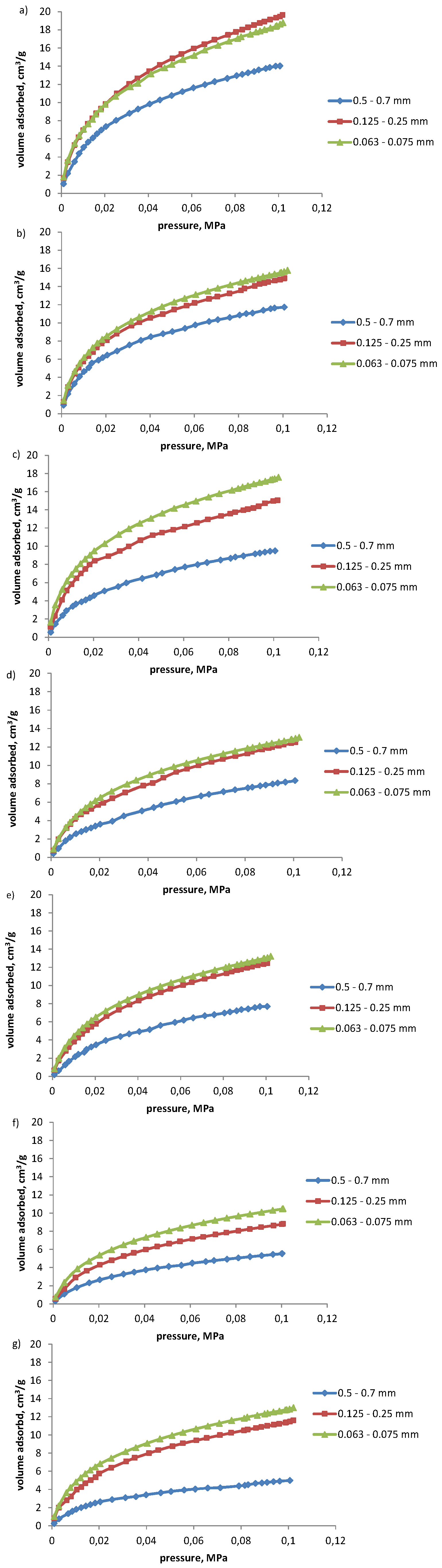
- The sorption capacity of coals with respect to propylene and acetylene depends on the grade of metamorphism, porosity, and chemical properties of coals. Coals of lower grade of metamorphism, high porosity, and high oxygen content tend to sorb higher volumes of the hydrocarbons discussed.
- Coals characterized by low or medium porosity and sorption capacity and high grade of metamorphism sorbed up to a few-fold higher volumes of acetylene than propylene. The difference in the volumes of propylene and acetylene sorbed was not so pronounced for coals of high sorption capacity. Higher amounts of acetylene sorbed result from its higher reactivity (triple bond between carbon atoms) and small kinetic diameter.
- The fragmentation of coal results in the increase in the amounts of propylene and acetylene sorbed, particularly noticeable for coals of higher grade of metamorphism, and low or medium sorption capacity. For such coals the increase of 3 to 6-fold in the volume of propylene sorbed with fragmentation of coal was observed, and of up to over 2-fold for acetylene. The structure of coals with a low degree of metamorphism is easily accessible for the molecules of hydrocarbons irrespective of coal grain size. The reduction in coal grain class results in the increase in its sorption capacity, and number of active centers on its surface. The highest increase in sorption capacity was observed with the change in the grain size range from 0.500–0.700 mm to 0.125–0.250 mm.
- The enhanced sorption of selected hydrocarbons, particularly with propylene, on fragmented coals may result in the underestimation of their contents in the self-ignition center and in the subsequent incorrect assessment of the development of the self-heating process. This makes the method of assessment of the development of the self-heating process based on the measurement of propylene and acetylene contents in mine air less suitable for coals of higher grades of metamorphism, in particular those susceptible to crushing.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dai, G.L. Study on the gaseous products in coal oxidation at low temperature. Coal-Mine Safe 2007, 1, 1–4. [Google Scholar]
- Singh, A.K.; Singh, R.V.K.; Singh, M.P.; Chandra, H.; Shukla, N.K. Mine fire gas indices and their application to Indian underground coal mine fires. Int. J. Coal Geol. 2007, 69, 192–204. [Google Scholar] [CrossRef]
- Smith, M.; Glasser, D. Spontaneous combustion of carbonaceous stockpiles. Part I: The relative importance of various intrinsic coal properties and properties of the reaction system. Fuel 2005, 84, 1151–1160. [Google Scholar] [CrossRef]
- Adamus, A.; Sancer, J.; Guranova, P.; Zubicek, V. An investigation of the factors associated with interpretation of mine atmosphere for spontaneous combustion in coal mines. Fuel Process. Technol. 2011, 92, 663–670. [Google Scholar] [CrossRef]
- Lu, P.; Liao, G.X.; Sun, J.H.; Li, P.D. Experimental research on index gas of the coal spontaneous at low-temperature stage. J. Loss Prev. Proc. 2004, 17, 243–247. [Google Scholar] [CrossRef]
- Taraba, B.; Pavelek, Z. Study of coal oxidation behavior in re-opened sealed heating. J. Loss Prev. Proc. 2016, 40, 433–436. [Google Scholar] [CrossRef]
- Xie, J.; Xue, S.; Cheng, W.; Wang, G. Early detection of spontaneous combustion of coal in underground coal mines with development of an ethylene enriching system. Int. J. Coal Geol. 2011, 85, 123–127. [Google Scholar] [CrossRef]
- Yuan, L.; Smith, A.C. CO and CO2 emissions from spontaneous heating of coal under different ventilation rates. Int. J. Coal Geol. 2011, 88, 24–30. [Google Scholar] [CrossRef]
- Dudzińska, A. Investigation of adsorption and desorption of acetylene on hard coal samples from Polish mines. Int. J. Coal Geol. 2014, 128–129, 24–31. [Google Scholar] [CrossRef]
- Dudzińska, A.; Howaniec, N.; Smoliński, A. Experimental study on sorption and desorption of propylene on Polish hard coals. Energy Fuel 2015, 29, 4850–4854. [Google Scholar] [CrossRef]
- Dudzińska, A.; Cygankiewicz, J. Analysis of adsorption tests of gases emitted in the coal self-heating process. Fuel Process. Technol. 2015, 137, 109–116. [Google Scholar] [CrossRef]
- Cygankiewicz, J.; Dudzińska, A.; Żyła, M. The effect of particle size of comminuted bituminous coal on low-temperature sorption of nitrogen and room temperature sorption of carbon dioxide. Przem. Chem. 2006, 85, 1505–1509. [Google Scholar]
- Cygankiewicz, J.; Dudzińska, A.; Żyła, M. The relation between the size of bituminous coal particles and the sorption of carbon monoxide. Gospod. Surowcami Miner. 2009, 25, 85–100. [Google Scholar]
- Żyła, M.; Kreiner, K. The effect of hard coal comminution on the sorption of vapours of polar and apolar substances. Arch. Min. Sci. 1993, 38, 41–50. [Google Scholar]
- Żyła, M.; Dudzińska, A.; Cygankiewicz, J. The influence of disintegration of hard coal varieties of different metamorphism grade on the amount of absorbed ethane. Arch. Min. Sci. 2013, 58, 449–463. [Google Scholar]
- Dudzińska, A. Examination of adsorption and desorption of ethylene on several samples of Polish hard coals. In Proceedings of the 14th International Multidisciplinary Scientific GeoConference on Science and Technologies in Geology, Exploration and Mining, Albena, Bulgaria, 17–26 June 2014. [Google Scholar]
- Polish Committee for Standardization (PCS). PN-G-04502:2014-11 Hard Coal and Lignite. Sampling and Preparation for Laboratory Tests. Basic Methods; Polish Committee for Standardization: Warsaw, Poland, 2014. [Google Scholar]
- Polish Committee for Standardization (PCS). PN-G-04571:1998 Solid Fuels. Determination of C, H, and N Content with Automatic Analyzers. Macro Method; Polish Committee for Standardization: Warsaw, Poland, 1998. [Google Scholar]
- Polish Committee for Standardization (PCS). PN-G-04560:1998 Solid Fuels. Determination of Moisture, Volatiles and Ash with the Application of Automatic Analyzer; Polish Committee for Standardization: Warsaw, Poland, 1998. [Google Scholar]
- Polish Committee for Standardization (PCS). PN-G-04516:1998 Solid Fuels. Determinations of Volatiles with Gravimetric Method; Polish Committee for Standardization: Warsaw, Poland, 1998. [Google Scholar]
- Polish Committee for Standardization (PCS). PN-G-04584:2001 Solid Fuels. Determination of Total Sulfur and Ash Sulphur with the Application of Automatic Analyzers; Polish Committee for Standardization: Warsaw, Poland, 2001. [Google Scholar]
- Polish Committee for Standardization (PCS). PN-ISO 7404-3:2001 Methods of Petrographic Analysis of Bituminous Coal and Anthracite. Method of Determination of Maceral Groups; Polish Committee for Standardization: Warsaw, Poland, 2001. [Google Scholar]
- Polish Committee for Standardization (PCS). PN-ISO 7404-5:2002 Methods of Petrographic Analysis of Bituminous Coal and Anthracite. Part 5: Microscopic Method; Polish Committee for Standardization: Warsaw, Poland, 2002. [Google Scholar]
- Saha, S.; Sharma, B.K.; Kumar, S.; Sahu, G.; Badhe, Y.P.; Tambe, S.S.; Kulkarni, B.D. Density measurements of coal samples by different probe gases and their interaction. Fuel 2007, 86, 1594–1600. [Google Scholar] [CrossRef]
- Chalmers, G.R.L.; Bustin, R.M. On the effects of petrographic composition on coalbed methane sorption. Int. J. Coal Geol. 2006, 69, 288–304. [Google Scholar] [CrossRef]
- Karacan, C.O.; Mitchell, G.D. Behaviour and effect of different coal microlithotypes during gas transport or carbon dioxide sequestration into coal seams. Int. J. Coal Geol. 2003, 53, 201–217. [Google Scholar] [CrossRef]
- Ozdemir, E.; Morsi, B.I.; Schroeder, K. CO2 adsorption capacity of argonne premium coals. Fuel 2004, 83, 1085–1094. [Google Scholar] [CrossRef]
- Dudzińska, A.; Żyła, M.; Cygankiewicz, J. Effect of disintegration of bituminous coal on the amount of sorbed ethane. Przem. Chem. 2014, 93, 206–211. [Google Scholar]
- Krzyżanowski, A.; Zarębska, K. The unpolar liquid vapour sorption on coal with various petrographic compositions. Gospod. Surowcami Miner. 2007, 23, 175–181. [Google Scholar]
- Hansen, A.E.; Hower, J.C. Notes on the relationship between microlithotype composition and Hardgrove grindability index for rank suites of Eastern Kentucky (Central Appalachian) coals. Int. J. Coal Geol. 2014, 131, 109–112. [Google Scholar] [CrossRef]
- Trimble, A.S.; Hower, J.C. Studies of the relationship between coal petrology and grinding properties. Int. J. Coal Geol. 2003, 54, 253–260. [Google Scholar] [CrossRef]
- Cygankiewicz, J. About determination of susceptibility of coals to spontaneous combustion using an adiabatic test method. Arch. Min. Sci. 2000, 45, 247–273. [Google Scholar]
| Coal Sample | Vitrinite Reflectance, % | Ultimate Analysis, wt.% Daf | Proximate Analysis, wt.%, Dry | Macerals and Minerals, vol. % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbon | Hydrogen | Nitrogen | Oxygen | Moisture | Ash | Volatiles | Vitrinite | Inertinite | Liptinite | Minerals | ||
| 1 | 0.51 | 77.69 | 4.53 | 1.17 | 15.18 | 11.11 | 14.45 | 38.14 | 67 | 28 | 5 | 11 |
| 2 | 0.71 | 83.20 | 5.07 | 0.96 | 10.45 | 3.08 | 4.23 | 31.80 | 59 | 36 | 8 | 3 |
| 3 | 0.70 | 84.39 | 5.29 | 1.14 | 9.01 | 3.39 | 2.65 | 37.91 | 60 | 30 | 10 | 1 |
| 4 | 0.92 | 88.45 | 4.81 | 1.59 | 4.81 | 1.75 | 3.01 | 28.47 | 73 | 20 | 7 | 1 |
| 5 | 1.01 | 86.89 | 4.83 | 1.27 | 6.82 | 0.60 | 8.92 | 30.87 | 91 | 8 | 1 | 4 |
| 6 | 0.89 | 84.07 | 4.44 | 1.40 | 9.99 | 1.19 | 7.69 | 32.94 | 67 | 31 | 2 | 4 |
| 7 | 0.78 | 84.34 | 3.99 | 1.52 | 7.49 | 1.85 | 14.18 | 35.58 | 60 | 31 | 9 | 14 |
| Sample | Pore Diameter Range: 5–7500 nm | SBET, m2/g | SD-R, m2/g | DR Micropore Volume, cm3/g | |
|---|---|---|---|---|---|
| Porosity, % | Pore Volume, cm3/g | ||||
| 1 | 13.55 | 0.115 | 18.95 | 170.12 | 0.068 |
| 2 | 4.05 | 0.031 | 1.54 | 140.43 | 0.056 |
| 3 | 3.47 | 0.028 | 0.75 | 160.51 | 0.064 |
| 4 | 2.81 | 0.022 | 0.43 | 115.78 | 0.046 |
| 5 | 2.39 | 0.018 | 0.36 | 120.21 | 0.048 |
| 6 | 1.86 | 0.014 | 0.48 | 90.44 | 0.036 |
| 7 | 2.37 | 0.018 | 0.66 | 103.18 | 0.041 |
| Sample | V1, cm3/g | V2, cm3/g | V3, cm3/g | V2/V1 | V3/V2 | V3/V1 |
|---|---|---|---|---|---|---|
| 1 | 14.92 | 16.52 | 17.63 | 1.1 | 1.1 | 1.2 |
| 2 | 9.03 | 11.29 | 13.06 | 1.3 | 1.2 | 1.4 |
| 3 | 3.44 | 8.84 | 13.64 | 2.6 | 1.5 | 4.0 |
| 4 | 3.82 | 8.95 | 12.38 | 2.3 | 1.4 | 3.2 |
| 5 | 3.69 | 8.68 | 12.49 | 2.4 | 1.4 | 3.4 |
| 6 | 2.58 | 5.38 | 9.96 | 2.1 | 1.9 | 3.9 |
| 7 | 1.55 | 5.63 | 9.78 | 3.6 | 1.7 | 6.3 |
| Sample | V1, cm3/g | V2, cm3/g | V3, cm3/g | V2/V1 | V3/V2 | V3/V1 |
|---|---|---|---|---|---|---|
| 1 | 14.03 | 19.46 | 18.64 | 1.4 | 1.0 | 1.3 |
| 2 | 11.98 | 14.94 | 15.64 | 1.2 | 1.0 | 1.3 |
| 3 | 9.50 | 14.96 | 17.44 | 1.6 | 1.2 | 1.8 |
| 4 | 8.33 | 12.52 | 12.91 | 1.5 | 1.0 | 1.5 |
| 5 | 7.67 | 12.47 | 13.07 | 1.6 | 1.0 | 1.7 |
| 6 | 5.56 | 8.80 | 10.49 | 1.6 | 1.2 | 1.9 |
| 7 | 4.99 | 11.47 | 12.85 | 2.3 | 1.1 | 2.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudzińska, A.; Howaniec, N.; Smoliński, A. Effect of Coal Grain Size on Sorption Capacity with Respect to Propylene and Acetylene. Energies 2017, 10, 1919. https://doi.org/10.3390/en10111919
Dudzińska A, Howaniec N, Smoliński A. Effect of Coal Grain Size on Sorption Capacity with Respect to Propylene and Acetylene. Energies. 2017; 10(11):1919. https://doi.org/10.3390/en10111919
Chicago/Turabian StyleDudzińska, Agnieszka, Natalia Howaniec, and Adam Smoliński. 2017. "Effect of Coal Grain Size on Sorption Capacity with Respect to Propylene and Acetylene" Energies 10, no. 11: 1919. https://doi.org/10.3390/en10111919
APA StyleDudzińska, A., Howaniec, N., & Smoliński, A. (2017). Effect of Coal Grain Size on Sorption Capacity with Respect to Propylene and Acetylene. Energies, 10(11), 1919. https://doi.org/10.3390/en10111919






