Development of an Energy Biorefinery Model for Chestnut (Castanea sativa Mill.) Shells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Crude and Pretreated CS
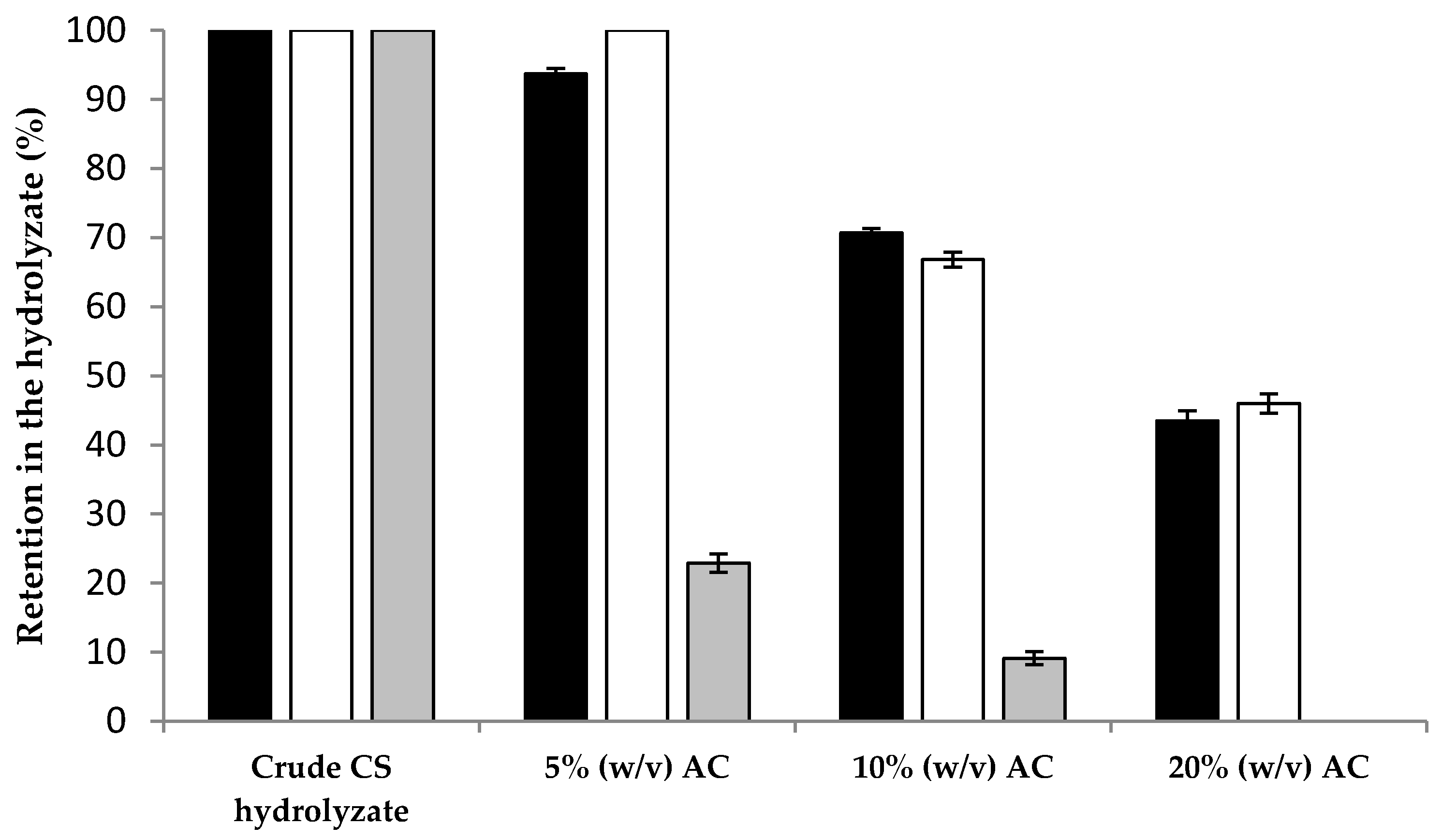
2.2. Saccharification of CS and Detoxification with Activated Charcoal
2.3. Desorption of Phenolic Compounds from Activated Charcoal and Radical Scavenging Activity
2.4. Fermentative Hydrogen and Butyrate Production
2.5. CS Biorefinery Model
- ENERGY 1: Biomass can be converted into energy (heat or electricity) through combustion, a process which is the most widely applied because of its low cost. The gross calorific values of chestnut shell and chestnut shell biochar are 15.49 and 25.86 MJ/kg, respectively [56]. Although it is expected that the chemical composition of spent CS, namely the final residue obtained after pretreatment and saccharification of CS, is somewhat different from crude CS or CS biochar, its use can be taken in consideration for energy production. As spent CS represent 32.5% (w/w) of the initial CS biomass (Figure 3), about 6100–9140 tons/year could be obtained from the total shell waste solid residue produced in 2014 in the Mediterranean basin.
- ENERGY 2: The price of conventionally produced H2 in 2016 averaged 4.0 USD/kg H2 in the USA, whereas the costs for the fermentative H2 production are currently estimated on 578 USD/kg H2 [57]. According to this reference, the high costs of the fermentative process are associated with a low molar yield and a low concentration of carbohydrates in the fermentation broth, which is adequate for research scale but not to scale-up to an industrial process. In fact, assuming the production yield obtained in the present work (63.9 mL H2/g), the total amount of CS generated in 2014 in the Mediterranean region would only correspond to 106,975–160,463 kg of H2. The feedstock cost and the capital cost for the industrial production are also critical to the high projected cost of hydrogen generated via DF of biomass. However, in the case of CS as waste biomass, the negative feedstock cost would impact positively on the process economic balance, so as the co-production of bioactive molecules. Considering an optimized scenario modeled with a fermentation broth concentration of 300 g/L and the conversion of excess lignin and biogas to thermal energy and further to electrical energy, a more competitive cost of 3.78 to 5.47 USD/kg H2 can be achieved [57].
- PRODUCT 1: Phenolic compounds belong to the wide class of phytonutrients, and find application in a variety of sectors such as food, feed, pharmaceutical and cosmetic industry. As they originate from natural sources, the diversity of biomolecules that can be obtained and the respective prices vary greatly, also depending on their purity and biological activity. The price of gallic and ellagic acids, two phenolic compounds present in chestnut extracts, is approximately 645 and 52,000 USD/kg, respectively (Sigma-Aldrich Company, Milano, Italy). The market of phenolic compounds is much diversified as they can be commercialized either as pure compounds or mixture of natural origin. In 2014, the global market of phytonutrients was estimated at 3.05 Billion USD and it is foreseen to reach 4.63 Billion USD within 2020, with Europe expecting to be the fastest-growing market in the near future [58]. As the amount of the phenolic compounds recovered from the hydrolyzate corresponds to the 0.74% (w/w) of the crude CS, it would be expected that about 138,600–207,900 kg/year could be obtained from CS.
- PRODUCT 2: The price of butyric acid is currently approximately 2000–2500 (USD/ton) [53], which represents an attractive income when transposed to the potential butyrate production from CS. Due to the variety of industrial applications for this organic acid, to the need to set aside petrochemicals based production processes, along with the newly explored pathways for the production of drop-in biofuels, the market size for butyrate was estimated in 30,000 (ton/year) in 2015 and in general “green” volatile fatty acids production exhibits a tendency to expand [59]. Using the butyrate production yield obtained in the present work (10.7 mM) and the amount of shell residues generated by the Mediterranean chestnut industry in 2014 (18,750–28,125 tons), it is possible to estimate a potential of 353,541–530,312 kg for butyrate production.
- REUSE 1: To become economically attractive, a detoxification process based on the use of an adsorbent matrix must foresee the reutilization of the adsorbent for additional cycles. As such, after the phenols desorption stage, AC can be reused for detoxification of a fresh hydrolyzate followed by phenolic compounds elution. This practice provides an environmental gain by avoidance of AC disposal. Additionally, the prospect of selectively recovering CS phenolic compounds with different antioxidant activities, as suggested by the RSA of the eluates from Elution I and II, is of major interest and deserves further investigation.
3. Materials and Methods
3.1. Chemicals
3.2. Biorefinery Stages
3.3. Pretreatment and Saccharification of CS
3.4. Detoxification of the CS Hydrolyzate
3.5. Desorption of Phenolic Compounds from the Charcoal
3.6. Fermentation of the Detoxified CS Hydrolyzate
3.7. Characterization of the samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Metger, J.O.; Huttermann, A. Sustainable global energy supply based on lignocellulosic biomass from afforestation of degraded areas. Naturwissenschaften 2009, 96, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Scarlat, N.; Martinov, M.; Dallemand, J.F. Assessment of the availability of agricultural crop residues in the European Union: Potential and limitations for bioenergy use. Waste Manag. 2010, 30, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Costa Lopes, A.M.; Roseiro, L.B.; Lukasik, R.B. Relevance of ionic liquids and biomass feedstocks for biomolecule extraction. In Ionic Liquids in the Biorefinery Concept: Challenges and Perspectives; Lukasik, R.B., Ed.; RSC Green Chemistry No. 36; The Royal Society of Chemistry: Cambridge, UK, 2016; pp. 121–163. [Google Scholar]
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from biomass: Technological versus environmental feasibility. A review. Biofuels Bioprod. Biorefin. 2017, 11, 195–214. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Cherubini, F.; Strømman, A.H. Production of biofuels and biochemicals from lignocellulosic biomass: Estimation of maximum theoretical yields and efficiencies using matrix algebra. Energy Fuels 2010, 24, 2657–2666. [Google Scholar] [CrossRef]
- Bragança, H. 2007 Chestnut Blight in Portugal: Spread and Populational Structure of Cryphonectria parasitica. Ph.D. Thesis, Faculty of Sciences of Lisbon, Lisbon University, Lisbon, Portugal, 2007. [Google Scholar]
- FAOSTAT, Food and Agriculture Organization of the United States. Available online: http://www.fao.org/faostat/en/#data (accessed on 26 September 2017).
- Economic Study for the Development of the Chestnut Sector, Portuguese Forestry Forum, Report Project SIAC 02/23070/2011. 2011. Available online: http://forumflorestal.pt/wp-content/uploads/2012/04/Relatório-Global-Castanha-versão-impressão.pdf (accessed on 26 September 2017).
- Vázquez, G.; González-Alvarez, J.; Santos, J.; Freire, M.S.; Antorrena, G. Evaluation of potential applications for chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crops Prod. 2009, 29, 364–370. [Google Scholar] [CrossRef]
- Liberti, A.; Goretti, G.; Russo, M.V. PCDD and PCDF formation in the combustion of vegetable wastes. Chemosphere 1983, 12, 661–663. [Google Scholar] [CrossRef]
- Wakefield, J.C. HPA-CHaPD-004—A Toxicological Review on the Products of Combustion, Health Protection Agency Chemical Research Reports. 2010; ISBN 978-0-85951-663-1. Available online: https://www.gov.uk/government/publications/combustion-products-a-toxicological-review (accessed on 29 August 2017).
- De Vasconcelos, M.C.; Bennett, R.N.; Rosa, E.A.; Ferreira-Cardoso, J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus). Ind. Crops Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-product through eco-friendly extraction methods. Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.S.; Lee, N.-K.; Na, D.S.; Yu, H.H.; Paik, H.-D. Comparative analysis of the antioxidant and anticancer activities of chestnut inner shell extracts prepared with various solvents. J. Sci. Food Agric. 2015, 96, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Maurelli, L.; Ionata, E.; La Cara, F.; Morana, A. Chestnut shell as unexploited source of fermentable sugars: Effect of different pretreatment methods on enzymatic saccharification. Appl. Biochem. Biotechnol. 2013, 170, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathana, P.; Kumar, G.; Mudhoo, A.; Rene, E.R.; Saratale, G.D.; Kobayashi, T.; Xu, K.; Kim, S.-H.; Kim, D.-H. Fermentative hydrogen production using lignocellulose biomass: An overview of pre-treatment methods, inhibitor effects and detoxification experiences. Renew. Sustain. Energy Rev. 2017, 77, 28–42. [Google Scholar] [CrossRef]
- Zhang, J.; Zang, L. Enhancement of biohydrogen production from brewers’ spent grain by calcined-red mud pretreatment. Bioresour. Technol. 2016, 209, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Fang, Z.; Chin, S.-X.; Tian, X.-F.; Su, T.-C. Biohydrogen production from hydrolysates of selected tropical biomass wastes with Clostridium butyricum. Sci. Rep. 2016, 6, 27205. [Google Scholar] [CrossRef] [PubMed]
- Schönicke, P.; Shahab, R.; Hamann, R.; Kamm, B. Microbial life on green biomass and their use for production of platform chemicals. In Microorganisms in Biorefineries (Microbiology Monographs 26); Kamm, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-45209-7. [Google Scholar]
- Zacharof, M.-P.; Vouzelaud, C.; Mandale, S.J.; Lovitt, R.W. Valorization of spent anaerobic digester effluents through production of platform chemicals using Clostridium butyricum. Biomass Bioenergy 2015, 81, 294–303. [Google Scholar] [CrossRef]
- Jha, A.K.; Li, J.; Yuan, Y.; Baral, N.; Ai, B. A review on bio-butyric acid production and its optimization. Int. J. Agric. Biol. 2014, 16, 1019–1024. [Google Scholar]
- Tai, J.; Adav, S.S.; Su, A.; Lee, D.-J. Biological hydrogen production from phenol-containing wastewater using Clostridium butyricum. Int. J. Hydrog. Energy 2010, 35, 13345–13349. [Google Scholar] [CrossRef]
- Quéméneur, M.; Hamelin, J.; Barakat, A.; Steyer, J.-P.; Carrère, H.; Trably, E. Inhibition of fermentative hydrogen production by lignocellulose-derived compounds in mixed cultures. Int. J. Hydrog. Energy 2012, 37, 3150–3159. [Google Scholar] [CrossRef]
- Ham, J.-S.; Kim, H.-Y.; Lim, S.-T. Antioxidant and deodorizing activities of phenolic components in chestnut inner shell extracts. Ind. Crops Prod. 2015, 73, 99–105. [Google Scholar] [CrossRef]
- Li, S.; Chen, G.; Zhang, C.; Wu, M.; Wu, S.; Liu, Q. Research progress of natural antioxidants in foods for thetreatment of diseases. Food Sci. Hum. Wellness 2014, 3, 110–116. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of Phenolic Compounds for Food Preservation: Food Additive and Active Packaging. In Phenolic Compounds—Biological Activity; Soto-Hernández, M., Ed.; In Tech: Rijeka, Croatia, 2017. [Google Scholar]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.; Andersson, J.; Gorton, L.; Larsson, S.; Nilvebrant, N.O.; Jönsson, L.J. Effect of different forms of alkali treatment on specific fermentation inhibitors and on the fermentability of lignocellulose hydrolysates for production of fuel ethanol. J. Agric. Food Chem. 2002, 50, 5318–5325. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, M.; Cantarella, L.; Gallifuoco, A.; Spera, A.; Alfani, F. Comparison of different detoxification methods for steam-exploded poplar wood as a substrate for the bioproduction of ethanol in SHF and SSF. Proc. Biochem. 2004, 39, 1533–1542. [Google Scholar] [CrossRef]
- Guo, X.; Cavka, A.; Jönsson, L.J.; Hong, F. Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb. Cell Fact. 2013, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Kapoor, R.K.; Singh, A.; Kuhad, R.C. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour. Technol. 2007, 98, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Schroyen, M.; Van Hulle, S.W.H.; Holemans, S.; Vervaeren, H.; Raes, K. Laccase enzyme detoxifies hydrolysates and improves biogas production from hemp straw and miscanthus. Bioresour. Technol. 2017, 244, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Adeboye, P.T.; Bettiga, M.; Olsson, L. The chemical nature of phenolic compounds determines their toxicity and induces distinct physiological responses in Saccharomyces cerevisiae in lignocellulose hydrolysates. AMB Express 2014, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Nitiema, L.W.; Savadogo, A.; Simpore, J.; Dianou, D.; Traore, A.S. In vitro antimicrobial activity of some phenolic compounds (Coumarin and Quercetin) against gastroenteritis bacterial strains. Int. J. Microbiol. Res. 2012, 3, 183–187. [Google Scholar] [CrossRef]
- Samad, N.B.; Debnath, T.; Hasnat, M.A.; Pervin, M.; Kim, D.H.; Jo, J.E.; Park, R.S.; Lim, B.O. Phenolic contents, antioxidant and anti-inflammatory activities of Asparagus cochinchinensis (Loureiro) Merrill. J. Food Biochem. 2014, 38, 83–91. [Google Scholar] [CrossRef]
- Ena, A.; Pintucci, C.; Carlozzi, P. The recovery of polyphenols from olive mill waste using two adsorbing vegetable matrices. J. Biotechnol. 2012, 157, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, B. Adsorption and desorption of phenol on activated carbon and a comparison of isotherm models. J. Hazard. Mater. 2006, 129, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, R.; Rehan, A.H. A study of the adsorption of phenol by activated carbon from aqueous solutions. Turk. J. Chem. 2002, 26, 357–361. [Google Scholar]
- Streicher, J.; Ruhl, A.S.; Gnirß, R.; Jekel, M. Where to dose powdered activated carbon in a wastewater treatment plant for organic micro-pollutant removal. Chemosphere 2016, 156, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-C.; Lu, W.-C.; Chen, C.-Y.; Chang, J.-S. Dark fermentative hydrogen production from enzymatic hydrolysate of xylan and pretreated rice straw by Clostridium butyricum CGS5. Bioresour. Technol. 2010, 101, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Ortigueira, J.; Pinto, T.; Gouveia, L.; Moura, P. Production and storage of biohydrogen during sequential batch fermentation of Spirogyra hydrolyzate by Clostridium butyricum. Energy 2015, 88, 528–536. [Google Scholar] [CrossRef]
- Beckers, L.; Masset, J.; Hamilton, C.; Delvigne, F.; Toye, D.; Crine, M.; Thonart, P.; Hiligsmann, S. Investigation of the links between mass transfer conditions, dissolved hydrogen concentration and biohydrogen production by the pure strain Clostridium butyricum CWBI1009. Biochem. Eng. J. 2015, 98, 18–28. [Google Scholar] [CrossRef]
- Han, H.; Wei, L.; Liu, B.; Yang, H.; Shen, J. Optimization of biohydrogen production from soybean straw using anaerobic mixed bacteria. Int. J. Hydrog. Energy 2012, 37, 13200–13208. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Periyasamy, S.; Kim, S.H.; Nemestóthy, N.; Bélafi-Bakó, K. Lignocellulose biohydrogen: Practical challenges and recent progress. Renew. Sustain. Energy Rev. 2015, 44, 728–737. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.R.; Bonk, F.; Thomsen, M.H.; Schmidt, J.E. Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev. Environ. Sci. Bio/Technol. 2015, 14, 473–498. [Google Scholar] [CrossRef]
- Dwidar, M.; Park, J.-Y.; Mitchell, R.J.; Sang, B.-I. The future of butyric acid in industry. Sci. World J. 2012, 471417. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, C.; Heeres, A.S.; van der Wielen, L.A.M.; Straathof, A.J.J. Simultaneous clostridial fermentation, lipase-catalyzed esterification, and ester extraction to enrich diesel with butyl butyrate. Biotechnol. Bioeng. 2013, 110, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.; Valdez-Vazquez, I.; Saratale, G.D.; Saratale, R.G.; Silva, C.; Ortigueira, J. Dark fermentative hydrogen production: From concepts to a sustainable production. In Microbial Fuels: Technologies and Applications; Harzevili, F.D., Hiligsmann, S., Eds.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Özçimen, D.; Ersoy-Meriçboyu, A. A study on the carbonization of grapeseed and chestnut shell. Fuel Process. Technol. 2008, 89, 1041–1046. [Google Scholar] [CrossRef]
- James, B.D.; DeSantis, D.A.; Moton, J.M.; Houchins, C. II.A.1 Hydrogen Pathways Analysis for Solid Oxide Fuel Cell (SOFC) and Dark Fermentation. Available online: https://www.hydrogen.energy.gov/pdfs/progress15/ii_a_1_james_2015.pdf (accessed on 26 September 2017).
- Report Phytonutrients Market by Type (Carotenoids, Phytosterols, Flavonoids, Phenolic Compounds, and Vitamin E), Application (Food & Beverage, Feed, Pharmaceutical, and Cosmetic), Source, & by Region—Global Trends and Forecast to 2020. June 2015. Available online: Marketsandmarkets.com (accessed on 26 September 2017).
- Zacharof, M.-P.; Lovitt, R.W. Recovery of volatile fatty acids (VFA) from complex waste effluents using membranes. Water Sci. Technol. 2014, 69, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Ortigueira, J.; Alves, L.; Gouveia, L.; Moura, P. Third generation biohydrogen production by Clostridium butyricum and adapted mixed cultures from Scenedesmus obliquus microalga biomass. Fuel 2015, 153, 128–134. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]



| Sample | Adsorbed Phenolic Compounds (mg GAE/L) | Desorbed Phenolic Compounds (mg GAE/L) | RSA (%) |
|---|---|---|---|
| CS hydrolyzate | - | - | 21.0 ± 1.1 |
| Activated charcoal | 525.8 ± 15.6 | - | - |
| Elution I | 182.5 ± 6.2 | 343.4 ± 13.2 | 51.8 ± 1.6 |
| Elution II | 156.2 ± 8.8 | 26.3 ± 2.3 | 26.0 ± 0.9 |
| Total (Elution I + II) | - | 369.6 ± 11.5 | - |
| Parameter | Result |
|---|---|
| Cumulative H2 production (mL/L) | 1598 ± 100 |
| Hydrogen yield (mL/g CS) | 63.9 ± 4.0 |
| H2/CO2 ratio (mol/mol) | 1.4 ± 0.1 |
| Acetate production (mM) | 13.3 ± 1.6 |
| Butyrate production (mM) | 10.7 ± 0.2 |
| Formate production (mM) | 6.9 ± 0.2 |
| Total sugars consumption (%) | 96.8 ± 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morana, A.; Squillaci, G.; Paixão, S.M.; Alves, L.; Cara, F.L.; Moura, P. Development of an Energy Biorefinery Model for Chestnut (Castanea sativa Mill.) Shells. Energies 2017, 10, 1504. https://doi.org/10.3390/en10101504
Morana A, Squillaci G, Paixão SM, Alves L, Cara FL, Moura P. Development of an Energy Biorefinery Model for Chestnut (Castanea sativa Mill.) Shells. Energies. 2017; 10(10):1504. https://doi.org/10.3390/en10101504
Chicago/Turabian StyleMorana, Alessandra, Giuseppe Squillaci, Susana M. Paixão, Luís Alves, Francesco La Cara, and Patrícia Moura. 2017. "Development of an Energy Biorefinery Model for Chestnut (Castanea sativa Mill.) Shells" Energies 10, no. 10: 1504. https://doi.org/10.3390/en10101504







