Effect of Thermal-Electric Cross Coupling on Heat Transport in Nanofluids
Abstract
:1. Introduction
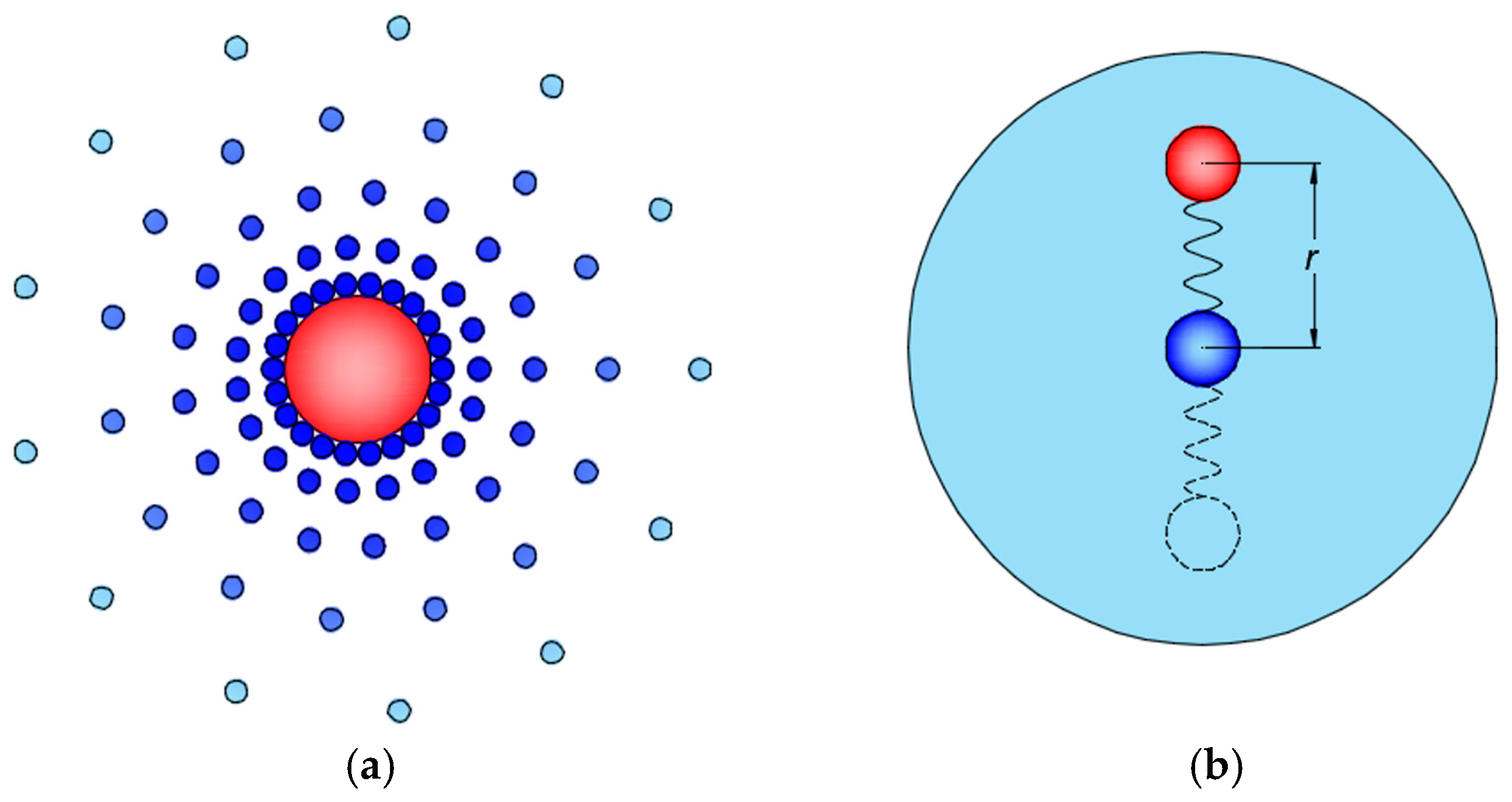
2. Model of Nanofluids
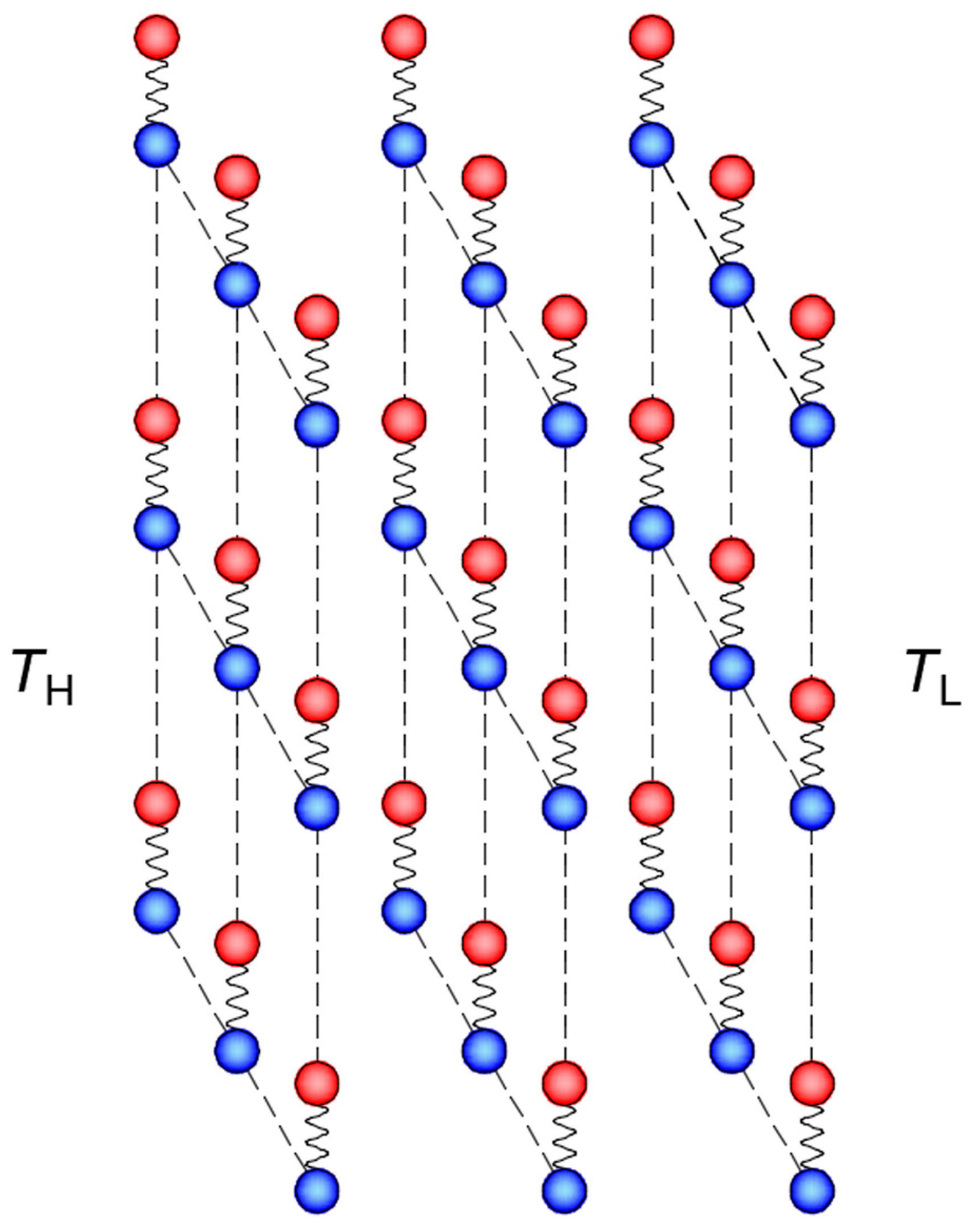
3. Cross Coupling of Thermal Motion and Electric Field
3.1. Effect of Electric Field on Heat Transport
3.2. Thermal Motion of Magnetic Nanoparticles
4. Influence Factors of Heat Transport in Nanofluids
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| Symbols | |
| radius of nanoparticle (m) | |
| concentration of species i (mol/m3) | |
| intensity of the electric field (N/C) | |
| kinetic energy (J) | |
| average kinetic energy (J) | |
| induced frequency (Hz) | |
| intrinsic frequency (Hz) | |
| Faraday constant (C/mol) | |
| Boltzmann constant (J/K) | |
| distance to the center of diffusion layer (m) | |
| distance between two nanoparticles (m) | |
| mass of a nanoparticle (kg) | |
| electric moment (C·m) | |
| charge quantity (C) | |
| vibration displacement (m) | |
| induced vibration displacement (m) | |
| intrinsic vibration displacement (m) | |
| vibration displacement (m) | |
| universal gas constant (J/(mol·K)) | |
| thermodynamic temperature (K) | |
| transient temperature (K) | |
| temperature difference (K) | |
| velocity (m/s) | |
| maximum velocity (m/s) | |
| average potential energy (J) | |
| chemical valence of species i (-) | |
| Greek Symbols | |
| dielectric constant (F/m) | |
| zeta potential (V) | |
| Debye length (m) | |
| angular velocity (rad/s) | |
| Subscript | |
| 1 | high-temperature nanoparticle |
| 2 | low-temperature nanoparticle |
References
- Goyal, P.; Baredar, P.; Mittal, A.; Siddiqui, A.R. Adsorption refrigeration technology–An overview of theory and its solar energy applications. Renew. Sustain. Energy Rev. 2016, 53, 1389–1410. [Google Scholar] [CrossRef]
- Koroneos, C.; Rovas, D. Exergy analysis of geothermal electricity using the kalina cycle. Int. J. Exergy 2013, 12, 54–69. [Google Scholar] [CrossRef]
- Mills, D. Advances in solar thermal electricity technology. Sol. Energy 2004, 76, 19–31. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, C.-Y. A review of solar collectors and thermal energy storage in solar thermal applications. Appl. Energy 2013, 104, 538–553. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-T.; Wang, Y.-H. Numerical simulation of three-dimensional transient cooling application on a portable electronic device using phase change material. Int. J. Therm. Sci. 2012, 51, 155–162. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, N.; Peng, J.; Fang, X.; Gao, X.; Fang, Y. Preparation and thermal energy storage properties of paraffin/expanded graphite composite phase change material. Appl. Energy 2012, 91, 426–431. [Google Scholar] [CrossRef]
- Baby, R.; Balaji, C. Experimental investigations on phase change material based finned heat sinks for electronic equipment cooling. Int. J. Heat Mass Transf. 2012, 55, 1642–1649. [Google Scholar] [CrossRef]
- Eastman, J.A.; Phillpot, S.R.; Choi, S.U.S.; Keblinski, P. Thermal transport in nanofluids. Annu. Rev. Mater. Res. 2004, 34, 219–246. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Mujumdar, A.S. Heat transfer characteristics of nanofluids: A review. Int. J. Therm. Sci. 2007, 46, 1–19. [Google Scholar] [CrossRef]
- Ebrahimnia-Bajestan, E.; Moghadam, M.C.; Niazmand, H.; Daungthongsuk, W.; Wongwises, S. Experimental and numerical investigation of nanofluids heat transfer characteristics for application in solar heat exchangers. Int. J. Heat Mass Transf. 2016, 92, 1041–1052. [Google Scholar] [CrossRef]
- Fan, L.-W.; Li, J.-Q.; Li, D.-Y.; Zhang, L.; Yu, Z.-T.; Cen, K.-F. The effect of concentration on transient pool boiling heat transfer of graphene-based aqueous nanofluids. Int. J. Therm. Sci. 2015, 91, 83–95. [Google Scholar] [CrossRef]
- Raja, M.; Vijayan, R.; Dineshkumar, P.; Venkatesan, M. Review on nanofluids characterization, heat transfer characteristics and applications. Renew. Sustain. Energy Rev. 2016, 64, 163–173. [Google Scholar] [CrossRef]
- Taylor, R.; Coulombe, S.; Otanicar, T.; Phelan, P.; Gunawan, A.; Lv, W.; Rosengarten, G.; Prasher, R.; Tyagi, H. Small particles, big impacts: A review of the diverse applications of nanofluids. J. Appl. Phys. 2013, 113, 011301. [Google Scholar] [CrossRef]
- Nnanna, A.G.A. Experimental model of temperature-driven nanofluid. J. Heat Trans. 2007, 129, 697–704. [Google Scholar] [CrossRef]
- Visconti, P.; Primiceri, P.; Costantini, P.; Colangelo, G.; Cavalera, G. Measurement and control system for thermo-solar plant and performance comparison between traditional and nanofluid solar thermal collectors. Int. J. Smart Sens. Intell. Syst. 2016, 9, 1220–1242. [Google Scholar]
- Milanese, M.; Colangelo, G.; Creti, A.; Lomascolo, M.; Iacobazzi, F.; de Risi, A. Optical absorption measurements of oxide nanoparticles for application as nanofluid in direct absorption solar power systems—Part II: ZnO, CeO2, Fe2O3 nanoparticles behavior. Sol. Energy Mater. Sol. Cells 2016, 147, 321–326. [Google Scholar] [CrossRef]
- Milanese, M.; Colangelo, G.; Creti, A.; Lomascolo, M.; Iacobazzi, F.; de Risi, A. Optical absorption measurements of oxide nanoparticles for application as nanofluid in direct absorption solar power systems—Part I: Water-based nanofluids behavior. Sol. Energy Mater. Sol. Cells 2016, 147, 315–320. [Google Scholar] [CrossRef]
- Michaelides, E.E. Transport properties of nanofluids. A critical review. J. Non-Equilib. Thermodyn. 2013, 38, 1–79. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Thermophysical and electrokinetic properties of nanofluids—A critical review. Appl. Therm. Eng. 2008, 28, 2109–2125. [Google Scholar] [CrossRef]
- Azmi, W.H.; Sharma, K.V.; Mamat, R.; Najafi, G.; Mohamad, M.S. The enhancement of effective thermal conductivity and effective dynamic viscosity of nanofluids—A review. Renew. Sustain. Energy Rev. 2016, 53, 1046–1058. [Google Scholar] [CrossRef]
- Eastman, J.; Choi, U.; Li, S.; Thompson, L.; Lee, S. Enhanced thermal conductivity through the development of nanofluids. In Proceedings of the 1996 Fall meeting of the Materials Research Society (MRS), Boston, MA, USA, 2–6 December 1996.
- Chopkar, M.; Sudarshan, S.; Das, P.; Manna, I. Effect of particle size on thermal conductivity of nanofluid. Metall. Mater. Trans. A 2008, 39, 1535–1542. [Google Scholar] [CrossRef]
- Angayarkanni, S.A.; Philip, J. Review on thermal properties of nanofluids: Recent developments. Adv. Colloid Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef] [PubMed]
- Ozerinc, S.; Kakac, S.; Yazicioglu, A.G. Enhanced thermal conductivity of nanofluids: A state-of-the-art review. Microfluid. Nanofluid. 2009, 8, 145–170. [Google Scholar] [CrossRef]
- Mintsa, H.A.; Roy, G.; Nguyen, C.T.; Doucet, D. New temperature dependent thermal conductivity data for water-based nanofluids. Int. J. Therm. Sci. 2009, 48, 363–371. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; de Risi, A.; Laforgia, D. Results of experimental investigations on the heat conductivity of nanofluids based on diathermic oil for high temperature applications. Appl. Energy 2012, 97, 828–833. [Google Scholar] [CrossRef]
- Xuan, Y.M.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Gu, B.M.; Hou, B.; Lu, Z.X.; Wang, Z.L.; Chen, S.F. Thermal conductivity of nanofluids containing high aspect ratio fillers. Int. J. Heat Mass Transf. 2013, 64, 108–114. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, S.R.; Kim, D. Thermal conductivity of metal-oxide nanofluids: Particle size dependence and effect of laser irradiation. J. Heat Transf. 2007, 129, 298–307. [Google Scholar] [CrossRef]
- Beck, M.P.; Yuan, Y.; Warrier, P.; Teja, A.S. The thermal conductivity of alumina nanofluids in water, ethylene glycol, and ethylene glycol+ water mixtures. J. Nanopart. Res. 2010, 12, 1469–1477. [Google Scholar] [CrossRef]
- Beck, M.P.; Yuan, Y.; Warrier, P.; Teja, A.S. The thermal conductivity of aqueous nanofluids containing ceria nanoparticles. J. Appl. Phys. 2010, 107, 066101. [Google Scholar] [CrossRef]
- Hwang, Y.; Ahn, Y.; Shin, H.; Lee, C.; Kim, G.; Park, H.; Lee, J. Investigation on characteristics of thermal conductivity enhancement of nanofluids. Curr. Appl. Phys. 2006, 6, 1068–1071. [Google Scholar] [CrossRef]
- Shima, P.D.; Philip, J. Role of thermal conductivity of dispersed nanoparticles on heat transfer properties of nanofluid. Ind. Eng. Chem. Res. 2014, 53, 980–988. [Google Scholar] [CrossRef]
- Sundar, L.S.; Farooky, M.H.; Sarada, S.N.; Singh, M.K. Experimental thermal conductivity of ethylene glycol and water mixture based low volume concentration of Al2O3 and CuO nanofluids. Int. Commun. Heat Mass Transf. 2013, 41, 41–46. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U. Role of brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U. Effects of various parameters on nanofluid thermal conductivity. J. Heat Transf. 2007, 129, 617–623. [Google Scholar] [CrossRef]
- Beck, M.P.; Sun, T.; Teja, A.S. The thermal conductivity of alumina nanoparticles dispersed in ethylene glycol. Fluid Phase Equilib. 2007, 260, 275–278. [Google Scholar] [CrossRef]
- Evans, W.; Fish, J.; Keblinski, P. Role of brownian motion hydrodynamics on nanofluid thermal conductivity. Appl. Phys. Lett. 2006, 88, 093116. [Google Scholar] [CrossRef]
- Kim, B.H.; Beskok, A.; Cagin, T. Molecular dynamics simulations of thermal resistance at the liquid-solid interface. J. Chem. Phys. 2008, 129, 174701. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Alvarado, B.; Kumar, S.; Peterson, G.P. Solid-liquid thermal transport and its relationship with wettability and the interfacial liquid structure. J. Phys. Chem. Lett. 2016, 7, 3497–3501. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, D.; Chen, J.; Walther, J.H.; Giapis, K.P.; Angelikopoulos, P.; Koumoutsakos, P. Kapitza resistance between few-layer graphene and water: Liquid layering effects. Nano Lett. 2015, 15, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.Q.; Kim, B. Transport phenomena of water in molecular fluidic channels. Sci. Rep. 2016, 6, 33881. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, F.; Milanese, M.; Colangelo, G.; Lomascolo, M.; de Risi, A. An explanation of the Al2O3 nanofluid thermal conductivity based on the phonon theory of liquid. Energy 2016, 116, 786–794. [Google Scholar] [CrossRef]
- Keblinski, P.; Phillpot, S.; Choi, S.; Eastman, J. Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int. J. Heat Mass Transf. 2002, 45, 855–863. [Google Scholar] [CrossRef]
- Milanese, M.; Iacobazzi, F.; Colangelo, G.; de Risi, A. An investigation of layering phenomenon at the liquid-solid interface in Cu and CuO based nanofluids. Int. J. Heat Mass Transf. 2016, 103, 564–571. [Google Scholar] [CrossRef]
- Evans, W.; Prasher, R.; Fish, J.; Meakin, P.; Phelan, P.; Keblinski, P. Effect of aggregation and interfacial thermal resistance on thermal conductivity of nanocomposites and colloidal nanofluids. Int. J. Heat Mass Transf. 2008, 51, 1431–1438. [Google Scholar] [CrossRef]
- Kimura, K.; Takashima, S.; Ohshima, H. Molecular approach to the surface potential estimate of thiolate-modified gold nanoparticles. J. Phys. Chem. B 2002, 106, 7260–7266. [Google Scholar] [CrossRef]
- Li, X.; Zhu, D.; Wang, X.; Wang, N.; Gao, J.; Li, H. Thermal conductivity enhancement dependent pH and chemical surfactant for Cu-H2O nanofluids. Thermochim. Acta 2008, 469, 98–103. [Google Scholar] [CrossRef]
- Smith, J.S.; Bedrov, D.; Smith, G.D. A molecular dynamics simulation study of nanoparticle interactions in a model polymer-nanoparticle composite. Compos. Sci. Technol. 2003, 63, 1599–1605. [Google Scholar] [CrossRef]
- Izvekov, S.; Violi, A.; Voth, G.A. Systematic coarse-graining of nanoparticle interactions in molecular dynamics simulation. J. Phys. Chem. B 2005, 109, 17019–17024. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Fichthorn, K.A. Molecular-dynamics simulation of forces between nanoparticles in a lennard-jones liquid. J. Chem. Phys. 2003, 119, 9745–9754. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Cao, D.; Zhang, L.; Guo, Z. Nanoparticle dispersion and aggregation in polymer nanocomposites: Insights from molecular dynamics simulation. Langmuir 2011, 27, 7926–7933. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Peterson, G. Experimental investigation of temperature and volume fraction variations on the effective thermal conductivity of nanoparticle suspensions (nanofluids). J. Appl. Phys. 2006, 99, 084314. [Google Scholar] [CrossRef]
- Chon, C.H.; Kihm, K.D.; Lee, S.P.; Choi, S.U. Empirical correlation finding the role of temperature and particle size for nanofluid (Al2O3) thermal conductivity enhancement. Appl. Phys. Lett. 2005, 87, 3107. [Google Scholar] [CrossRef]


© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Z.; Wang, L. Effect of Thermal-Electric Cross Coupling on Heat Transport in Nanofluids. Energies 2017, 10, 123. https://doi.org/10.3390/en10010123
Kang Z, Wang L. Effect of Thermal-Electric Cross Coupling on Heat Transport in Nanofluids. Energies. 2017; 10(1):123. https://doi.org/10.3390/en10010123
Chicago/Turabian StyleKang, Zhanxiao, and Liqiu Wang. 2017. "Effect of Thermal-Electric Cross Coupling on Heat Transport in Nanofluids" Energies 10, no. 1: 123. https://doi.org/10.3390/en10010123




