Acute Stress and Anxiety in Medical Residents on the Emergency Department Duty
Abstract
:1. Introduction
2. Methods and Subjects
2.1. Population and Study Sample
2.2. Data Collection
- (1)
- Data were gathered on sex, age, hours of sleep on study days, mean number of hours on ED duty/month, and year of residency (to analyze possible interferences with cortisol release patterns) [25].
- (2)
- Saliva samples were collected in Salivette tubes (Sarstedt International, Nümbrecht, Germany), centrifuged at 2555× g. for 8 min and stored at −22 °C until further analysis. Salivary cortisol concentrations (µg/dL) were measured using an electrochemiluminescence immunoassay (Elecsys Cortisol test kit, Roche Diagnostics, Barcelona, Spain) on a Cobas c8000 analyzer (Roche Diagnostics). Salivary cortisol is a valid and reliable marker of the level of this hormone in plasma [16] and its collection is non-invasive, with minimal impact on daily life [25].
- (3)
2.3. Procedure
2.4. Ethical Considerations
2.5. Data Analysis
3. Results
3.1. Comparison of Salivary Cortisol between Regular Working Days and ED-Duty Days
3.2. Socio-Demographic Variables and Salivary Cortisol Release
3.3. Psychological Scores in Anxiety-State
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Le Fevre, M.; Matheny, J.; Kolt, G.S. Eustress, distress, and interpretation in occupational stress. J. Manag. Psychol. 2003, 18, 726–744. [Google Scholar] [CrossRef]
- Leung, M.-Y.; Chan, I.Y.S.; Cooper, C.L. Theories of Stress, in Stress Management in the Construction Industry; John Wiley and Sons, Ltd.: Chichester, UK, 2014. [Google Scholar] [CrossRef]
- Alonso, J.; Angermeyer, M.C.; Bernert, S.; Bruffaerts, R.; Brugha, T.S.; Bryson, H.; de Girolamo, G.; Graaf, R.; Demyttenaere, K.; Gasquet, I.; et al. Prevalence of mental disorders in Europe: Results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) Project. Acta Psychiatr. Scand. Suppl. 2004, 109, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sonnentag, S.; Fritz, C. Endocrinological processes associated with job stress: Catecholamine and cortisol responses to acute and chronic stressors. In Research in Organizational Stress and Wellbeing: Employee Health, Coping, and Methodologies; Perrewé, P.L., Ganster, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–59. [Google Scholar]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Kawachi, I. Work Stress as a Risk Factor for Cardiovascular Disease. Curr. Cardiol. Rep. 2015, 17, 630. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Lima, K.; Loureiro, S.R. Burnout, anxiety, depression, and social skills in medical residents. Psychol. Health Med. 2015, 20, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Angerer, P.; Petru, R.; Nowak, D.; Weigl, M. Workingconditions and depression in physicians. Dtsch. Med. Wochenschr. 2008, 133, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Buddeberg-Fischer, B.; Stamm, M.; Buddeberg, C.; Klaghofer, R. Anxiety and depression in residents—Results of a Swiss longitudinal study. Z. Psychosom. Med. Psychother. 2009, 55, 37–50. [Google Scholar] [PubMed]
- Dahlgren, A.; Kecklund, G.; Theorell, T.; Åkerstedt, T. Day-to-day variation in saliva cortisol—Relation with sleep, stress and self-rated health. Biol. Psychol. 2009, 82, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Jones, A.D.; Sun, K.; Neitzel, R.L. The association between noise, cortisol and heart rate in a small-scale gold mining community—A pilot study. Int. J. Environ. Res. Public Health 2015, 12, 9952–9966. [Google Scholar] [CrossRef] [PubMed]
- Bigert, C.; Bluhm, G.; Theorell, T. Saliva cortisol—A new approach in noise research to study stress effects. Int. J. Hyg. Environ. Health 2005, 208, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Lovell, B.; Moss, M.; Wetherell, M.A. With a little help from my friends: Psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res. Dev. Disabil. 2012, 33, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Kunz-Ebrecht, S.; Kirschbaum, C.; Marmot, M.; Steptoe, A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology 2004, 29, 516–528. [Google Scholar] [CrossRef]
- Taylor, M.K. Trait anxiety and salivary cortisol during free living and military stress. Aviat. Space Environ. Med. 2008, 79, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, S.S.; Kemeny, M.A. Acute stressors and cortisol response: A theoretical integration and synthesis of laboratory research. Psychol. Rev. 2004, 96, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; O’Connor, D.B.; Schaefer, A.; Talbot, D.; Hendrickx, H. The cortisol awakening response: Associations with trait anxiety and stress reactivity. Pers. Individ. Differ. 2011, 51, 123–127. [Google Scholar] [CrossRef]
- Sluiter, J.K.; van der Beek, A.J.; Frings-Dresen, M.H. Medical staff in emergency situations: Severity of patient status predicts stress hormone reactivity and recovery. Occup. Environ. Med. 2003, 60, 373–375. [Google Scholar] [CrossRef] [PubMed]
- González-Cabrera, J.; Fernández-Prada, M.; Molina-Ruano, R.; Blázquez, A.; Guillén-Solvas, J.; Peinado, J.M. Psychosocial risk at work, self-perceived stress and cortisol in saliva in a sample of emergency physicians in Granada. Emergencias 2012, 24, 101–106. [Google Scholar]
- González-Cabrera, J.; Fernández-Prada, M.; Iribar, C.; Peinado, J.M. Acute and chronic stress increase salivary cortisol: A study in the real-life setting of a national examination undertaken by medical graduates. Stress 2014, 17, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, Y.; Sakami, A.; Imamura, Y.; Tsuruta, T.; Egami, M.; Yamada, S. The effect of oral presentation on salivary 3-methoxy-4-hydroxy-phenylglycol (MHPG) and cortisol concentrations in training doctors: A preliminary study. Endocrine 2012, 42, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Dyrbye, L.N.; West, C.P.; Satele, D.; Boone, S.; Tan, L.; Sloan, J.; Shanafelt, T.D. Burnout among U.S. medical students, residents, and early career physicians relative to the general U.S. Popul. Acad. Med. 2014, 89, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Busireddy, K.R.; Miller, J.A.; Ellison, K.; Ren, V.; Qayyum, R.; Panda, M. Efficacy of interventions to reduce resident physician burnout: A systematic review. J. Grad. Med. Educ. 2017, 9, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Mata, D.A.; Ramos, M.A.; Bansal, N.; Khan, R.; Guille, C.; Angelantonio, E.; Sen, S. Prevalence of depression and depressive symptoms among resident physicians. JAMA 2015, 314, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Kudielka, B.M.; Hellhammer, D.H.; Wüst, S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 2009, 34, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R. Manual del Cuestionario de Ansiedad Estado/Rasgo (STAI); TEA Ediciones: Madrid, Spain, 1982. [Google Scholar]
- Guillén-Riquelme, A.; Buela-Casal, G. Actualización psicométrica y funcionamiento diferencial de los ítems en el state trait anxiety inventory (STAI). Psicothema 2011, 23, 510–515. [Google Scholar] [PubMed]
- Stone, A.A.; Schwartz, J.E.; Smyth, J.; Kirschbaum, C.; Cohen, S.; Hellhammer, D. Individual differences in the diurnal cycle of salivary free cortisol: A replication of flattened cycles for some individuals. Psychoendocrinology 2001, 26, 295–306. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for the computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Alobid, I.; De Pablo, J.; Mullol, J.; Centellas, S.; Parramon, G.; Carrasco, J.; Armario, A.; Bernal-Sprekelsen, M. Increased cardiovascular and anxiety outcomes but not endocrine biomarkers of stress during performance of endoscopic sinus surgery: A pilot study among novice surgeons. J. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Takahashi, T.; Shetty, V.; Yamaguchi, M. Patters of salivary cortisol levels can manifest work stress in emergency care providers. J. Physiol. Sci. 2012, 62, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fujimaru, C.; Okamura, H.; Kawasaki, M.; Kakuma, T.; Yoshii, C.; Matsuishi, T. Self-perceived work-related stress and its relation to salivary IgA, cortisol and 3-methoxy-4-hydroxyphenyl glycol levels among neonatal intensive care nurses. Stress Health 2012, 28, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Gaab, J.; Rohleder, N.; Nater, U.M.; Ehlert, U. Psychological determinants of the cortisol stress response: The role of anticipatory cognitive appraisal. Psychoneuroendocrinology 2005, 30, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Vedhara, K.; Perks, P.; Wilcock, G.K.; Lightman, S.L.; Shanks, N. Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. J. Neuroimmunol. 2000, 103, 84–92. [Google Scholar] [CrossRef]
- Fries, E.; Dettenborn, L.; Kirschbaum, C. The cortisol awakening response (CAR): Facts and future directions. Int. J. Psychophysiol. 2009, 72, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ritvanen, T.; Louhevaara, V.; Helin, P.; Väisänen, S.; Hänninen, O. Responses of the autonomic nervous system during periods of perceived high and low work stress in younger and older female teachers. Appl. Ergon. 2006, 37, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Weibel, L.; Gabrion, I.; Aussedat, M.; Kreutz, G. Work-related stress in an emergency medical dispatch center. Ann. Emerg. Med. 2003, 41, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Kirschbaum, C.; Prüssner, J.; Hellhammer, D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med. 1998, 14, 91–97. [Google Scholar] [CrossRef]
- Yu, Y.Z.; Shi, J.X. Relationship between levels of testosterone and cortisol in saliva and aggressive behaviors of adolescents. Biomed. Environ. Sci. 2009, 22, 44–49. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Othman, A.; Albanyan, A.; Al-Attas, O.S.; Alokail, M.S.; Sabico, S.; Chrousos, G.P. Perceived stress scores among Saudi students entering universities: A prospective study during the first year of university life. Int. J. Environ. Res. Public Health 2014, 11, 3972–3981. [Google Scholar] [CrossRef] [PubMed]
- Hamader, G.; Noehammer, E. Psychology of Well-Being: Theory, Perspectives and Practice; Nova Science Publishers: Hauppauge, NY, USA, 2013. [Google Scholar]
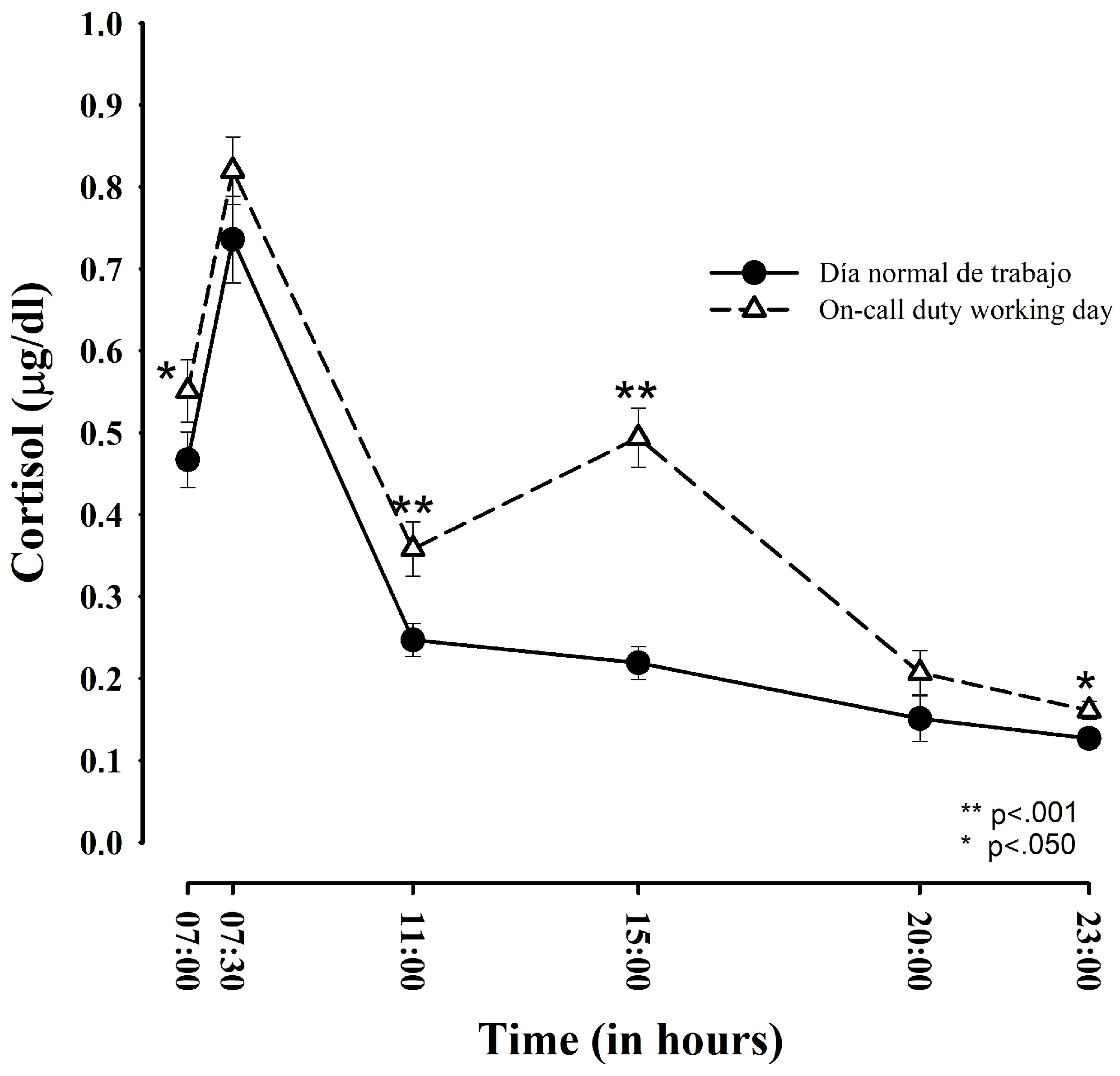

| Time points | Regular Work Day | ED-Duty Day | Student’s t-Test (t) | p Value | Effect Size (d) |
|---|---|---|---|---|---|
| Upon awakening | 0.467 (± 0.198) a 0.375 (± 0.127) b | 0.551 (± 0.221) 0.429 (± 0.136) | 2.033; (−0.108; −0.001) | 0.050 * | 0.41 |
| After 30 min | 0.736 (± 0.310) a 0.538 (± 0.167) b | 0.819 (± 0.279) 0.587 (± 0.154) | −1.635; (−0.109; 0.012) | 0.112 | 0.30 |
| At 11:00 h | 0.247 (± 0.116) a 0.217 (± 0.087) b | 0.358 (± 0.192) 0.298 (± 0.126) | −5.686; (−0.109; −0.051) | 0.001 * | 0.75 |
| At 15:00 h | 0.219 (± 0.119) a 0.193 (± 0.092) b | 0.494 (± 0.210) 0.392 (± 0.139) | −8.283; (−0.248; −0.151) | 0.001 * | 1.68 |
| At 20:00 h | 0.151 (± 0.109) a 0.137 (± 0.086) b | 0.207 (± 0.157) 0.181 (± 0.110) | −1.966; (−0.090; 0.001) | 0.058 | 0.44 |
| At 23:00 h | 0.127 (± 0.074) a 0.117 (± 0.064) b | 0.161 (± 0.102) 0.146 (± 0.081) | −2.040; (−0.057; −0.001) | 0.050 * | 0.40 |
| Total AUC | Bilateral Student’s t and Cohen’s d | CAR | Bilateral Student’s t and Cohen’s d | |
|---|---|---|---|---|
| Regular working day | 8.777 (± 3.811) a 2.214 (± 0.362) b | t = −7.959 p < 0.001 * (−0.513; −0.304) d = 1.15 | 0.2689 (± 0.238)
0.2213 (± 0.285) | t = −0.064 p < 0.950 (0.044; −0.093) d = 0.01 |
| Day on duty | 13.620 (± 5.199) a 2.623 (± 0.352) b | 0.2682 (± 0.214)
0.2242 (± 0.165) |
| Regular Working Day | ED-Duty Day | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| n = 11 | n = 24 | n = 11 | n = 24 | |
| STAI-State (comparison with data from Spielberger et al. [26]) | 19.08 (±5.07) | 20.17 (±5.24) | 23.42 (±5.93) | 25.70 (±5.82) |
| p = 0.344 | p = 0.009 * | p = 0.121 | p = 0.037 * | |
| t = −0.988 | t = −2.858 | t = 1.680 | t = 2.217 | |
| STAI-State (comparison with data from Guillén-Riquelme and Buela-Casal [27]) | 19.08 (±5.07) | 20.17 (±5.24) | 23.42 (±5.93) | 25.70 (±5.82) |
| p = 0.052 | p = 0.085 | p = 0.001 * | p = 0.001 * | |
| t = 2.180 | t = 1.805 | t = 4.408 | t = 6.936 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cabrera, J.M.; Fernández-Prada, M.; Iribar, C.; Molina-Ruano, R.; Salinero-Bachiller, M.; Peinado, J.M. Acute Stress and Anxiety in Medical Residents on the Emergency Department Duty. Int. J. Environ. Res. Public Health 2018, 15, 506. https://doi.org/10.3390/ijerph15030506
González-Cabrera JM, Fernández-Prada M, Iribar C, Molina-Ruano R, Salinero-Bachiller M, Peinado JM. Acute Stress and Anxiety in Medical Residents on the Emergency Department Duty. International Journal of Environmental Research and Public Health. 2018; 15(3):506. https://doi.org/10.3390/ijerph15030506
Chicago/Turabian StyleGonzález-Cabrera, Joaquín M., María Fernández-Prada, Concepción Iribar, Rogelio Molina-Ruano, María Salinero-Bachiller, and José M. Peinado. 2018. "Acute Stress and Anxiety in Medical Residents on the Emergency Department Duty" International Journal of Environmental Research and Public Health 15, no. 3: 506. https://doi.org/10.3390/ijerph15030506





