Atherogenic Index of Plasma Predicts Hyperuricemia in Rural Population: A Cross-Sectional Study from Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Lifestyle Factors
2.4. Blood Pressure Measurements
2.5. Anthropometric Measurements
2.6. Serum Analysis
2.7. Definition of Dyslipidemia
2.8. Definition of AIP
2.9. Definition of Hyperuricemia
2.10. Statistical Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, H.; Zhang, X.M.; Wang, Y.L.; Liu, B.C. Prevalence of hyperuricemia among Chinese adults: A national cross-sectional survey using multistage, stratified sampling. J. Nephrol. 2014, 27, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The national health and nutrition examination survey 2007–2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Trifiro, G.; Morabito, P.; Cavagna, L.; Ferrajolo, C.; Pecchioli, S.; Simonetti, M.; Bianchini, E.; Medea, G.; Cricelli, C.; Caputi, A.P.; et al. Epidemiology of gout and hyperuricaemia in Italy during the years 2005–2009: A nationwide population-based study. Ann. Rheum. Dis. 2013, 72, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Uaratanawong, S.; Suraamornkul, S.; Angkeaw, S.; Uaratanawong, R. Prevalence of hyperuricemia in Bangkok population. Clin. Rheumatol. 2011, 30, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Kivity, S.; Kopel, E.; Maor, E.; Abu-Bachar, F.; Segev, S.; Sidi, Y.; Olchovsky, D. Association of serum uric acid and cardiovascular disease in healthy adults. Am. J. Cardiol. 2013, 111, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Storhaug, H.M.; Norvik, J.V.; Toft, I.; Eriksen, B.O.; Lochen, M.L.; Zykova, S.; Solbu, M.; White, S.; Chadban, S.; Jenssen, T. Uric acid is a risk factor for ischemic stroke and all-cause mortality in the general population: A gender specific analysis from the Tromso study. BMC Cardiovasc. Disord. 2013, 13, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, E.; Baker, J.F.; Furst, D.E.; Schumacher, H.R. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006, 54, 2688–2696. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.J.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam study. Stroke 2006, 37, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; He, F.; Wu, X.; Peng, F.; Huang, F.; Yu, X. Relationship between serum uric acid and all-cause and cardiovascular mortality in patients treated with peritoneal dialysis. Am. J. Kidney Dis. 2014, 64, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Huang, L.; Song, M.; Song, Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: A meta-analysis of prospective studies. Atherosclerosis 2013, 231, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Koenig, W.; Baumert, J.; Doring, A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: The MONICA/KORA cohort study. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Chuang, S.Y.; Chen, H.J.; Yeh, W.T.; Pan, W.H. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: A Chinese cohort study. Arthritis Rheum. 2009, 61, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Li, J.; Xu, Y.X.; Wang, Y.L.; Luo, Y.Y.; Hu, D.Y.; Liu, W.J.; Yang, M.; Pi, L.; Wang, M.S.; et al. Predictive value of serum uric acid on cardiovascular disease and all-cause mortality in urban Chinese patients. Chin. Med. J. 2010, 123, 1387–1391. [Google Scholar] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- Dobiasova, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FERHDL). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Dobiasova, M.; Frohlich, J. The new atherogenic plasma index reflects the triglyceride and HDL-cholesterol ratio, the lipoprotein particle size and the cholesterol esterification rate: Changes during lipanor therapy. Vnitr. Lek. 2000, 46, 152–156. [Google Scholar] [PubMed]
- Njajou, O.T.; Kanaya, A.M.; Holvoet, P.; Connelly, S.; Strotmeyer, E.S.; Harris, T.B.; Cummings, S.R.; Hsueh, W.C. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the health, aging and body composition study. Diabetes Metab. Res. Rev. 2009, 25, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Dobiasova, M.; Urbanova, Z.; Samanek, M. Relations between particle size of HDL and LDL lipoproteins and cholesterol esterification rate. Physiol. Res. 2005, 54, 159–165. [Google Scholar] [PubMed]
- Onat, A.; Can, G.; Kaya, H.; Hergenc, G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J. Clin. Lipidol. 2010, 4, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic index of plasma (AIP): A marker of cardiovascular disease. Med. J. Islam Repub. Iran. 2015, 29, 240. [Google Scholar] [PubMed]
- Lippi, G.; Montagnana, M.; Luca Salvagno, G.; Targher, G.; Cesare Guidi, G. Epidemiological association between uric acid concentration in plasma, lipoprotein(a), and the traditional lipid profile. Clin. Cardiol. 2010, 33, E76–E80. [Google Scholar] [CrossRef] [PubMed]
- Tutal, E.; Sayin, B.; Ertugrul, D.T.; Ibis, A.; Sezer, S.; Ozdemir, N. Is there a link between hyperuricemia, morning blood pressure surge, and non-dipping blood pressure pattern in metabolic syndrome patients? Int. J. Nephrol. Renovasc. Dis. 2013, 6, 71–77. [Google Scholar] [PubMed]
- O’Brien, E.; Petrie, J.; Littler, W.; de Swiet, M.; Padfield, P.L.; O’Malley, K.; Jamieson, M.; Altman, D.; Bland, M.; Atkins, N. The British hypertension society protocol for the evaluation of automated and semi-automated blood pressure measuring devices with special reference to ambulatory systems. J. Hypertens. 1990, 8, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Raslova, K.; Dobiasova, M.; Hubacek, J.A.; Bencova, D.; Sivakova, D.; Dankova, Z.; Franekova, J.; Jabor, A.; Gasparovic, J.; Vohnout, B. Association of metabolic and genetic factors with cholesterol esterification rate in HDL plasma and atherogenic index of plasma in a 40 years old slovak population. Physiol. Res. 2011, 60, 785–795. [Google Scholar] [PubMed]
- Akbas, E.M.; Timuroglu, A.; Ozcicek, A.; Ozcicek, F.; Demirtas, L.; Gungor, A.; Akbas, N. Association of uric acid, atherogenic index of plasma and albuminuria in diabetes mellitus. Int. J. Clin. Exp. Med. 2014, 7, 5737–5743. [Google Scholar] [PubMed]
- Li, L.M.; Rao, K.Q.; Kong, L.Z.; Yao, C.H.; Xiang, H.D.; Zhai, F.Y.; Ma, G.S.; Yang, X.G.; Technical Working Group of China National Nutrition and Health Survey. A description on the Chinese national nutrition and health survey in 2002. Zhonghua Liu Xing Bing Xue Za Zhi 2005, 26, 478–484. [Google Scholar] [PubMed]
- Facchini, F.; Chen, Y.D.; Hollenbeck, C.B.; Reaven, G.M. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 1991, 266, 3008–3011. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Wietlisbach, V.; Bovet, P.; Shamlaye, C.; Riesen, W.; Paccaud, F.; Burnier, M. Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a developing country. BMC Public Health 2004, 4, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinahones, F.J.; Soriguer, F.J.; Collantes, E.; Perez-Lindon, G.; Sanchez Guijo, P.; Lillo, J.A. Decreased triglyceride levels with low calorie diet and increased renal excretion of uric acid in hyperuricaemic-hyperlipidaemic patients. Ann. Rheum. Dis. 1995, 54, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Chen, H.; Ren, J.Y.; Wang, L.; Luo, Y. Effects of micronized fenofibrate on lipid and uric acid metabolism in patients with hyperlipidemia. Beijing Da Xue Xue Bao 2009, 41, 541–544. [Google Scholar] [PubMed]
- Ishizaka, N.; Ishizaka, Y.; Toda, E.; Nagai, R.; Yamakado, M. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Olivieri, O.; Girelli, D.; Guarini, P.; Corrocher, R. Relationships between serum uric acid and lipids in healthy subjects. Prev. Med. 1996, 25, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Baliarsingh, S.; Sharma, N.; Mukherjee, R. Serum uric acid: Marker for atherosclerosis as it is positively associated with “atherogenic index of plasma”. Arch. Physiol. Biochem. 2013, 119, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen-Markkola, H.; Yki-Jarvinen, H. Hyperuricemia and insulin resistance. J. Clin. Endocrinol. Metab. 1994, 78, 25–29. [Google Scholar] [PubMed]

| Variables | Total (n = 11,345) | Normal (n = 10,241) | Hyperuricemia (n = 1104) | p-Value |
|---|---|---|---|---|
| Age (years) | 53.8 ± 10.6 | 53.7 ± 10.9 | 54.7 ± 11.0 | <0.01 |
| Man (%) | 5253 (46.3) | 4535 (44.3) | 718 (65.0) | <0.001 |
| Education Level | - | - | - | 0.337 |
| Low | 5760 (50.8) | 5101 (49.8) | 551 (49.9) | - |
| Middle | 4539 (38.4) | 4195 (41.0) | 428 (38.8) | - |
| High | 1046 (9.2) | 945 (9.2) | 125 (11.3) | - |
| Family Income (CNY/year) | - | - | - | 0.801 |
| ≤5000 | 1607 (14.2) | 1255 (12.3) | 149 (13.5) | - |
| 5000–20,000 | 6060 (53.4) | 5610 (54.8) | 583 (52.8) | - |
| >20,000 | 3678 (32.4) | 3376 (32.9) | 372 (33.7) | - |
| Smokers (%) | 4007 (35.3) | 3569 (34.9) | 438 (39.5) | <0.001 |
| Drinkers (%) | 2565 (22.6) | 2190 (21.4) | 375 (34.0) | <0.001 |
| Han (%) | 10,759 (94.8) | 9697 (94.7) | 1062 (96.2) | <0.05 |
| Others a (%) | 586 (5.2) | 544 (5.3) | 42 (3.8) | - |
| BMI (kg/m2) | 24.8 ± 3.7 | 24.6 ± 3.6 | 26.5 ± 3.9 | <0.001 |
| WC (cm) | 82.4 ± 9.8 | 81.7 ± 9.6 | 88.4 ± 9.8 | <0.001 |
| Serum Indicators | - | - | - | - |
| Uric Acid (mg/dL) | 291.9 ± 84.8 | 273.9 ± 64.5 | 458.5 ± 68.0 | <0.001 |
| SBP (mmHg) | 141.7 ± 23.4 | 140.3 ± 23.0 | 153.2 ± 24.0 | <0.001 |
| DBP (mmHg) | 82.0 ± 11.8 | 81.6 ± 11.6 | 85.5 ± 12.5 | <0.001 |
| LDL (mmol/L) | 2.9 ± 0.8 | 2.9 ± 0.8 | 3.1 ± 0.9 | <0.001 |
| HDL (mmol/L) | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.4 | <0.001 |
| TG (mmol/L) | 1.6 ± 1.5 | 1.5 ± 1.3 | 2.5 ± 2.5 | <0.001 |
| TC (mmol/L) | 5.2 ± 1.1 | 5.2 ± 1.1 | 5.6 ± 1.2 | <0.001 |
| FPG (mmol/L) | 5.9 ± 1.6 | 5.9 ± 1.7 | 6.1 ± 1.5 | <0.001 |
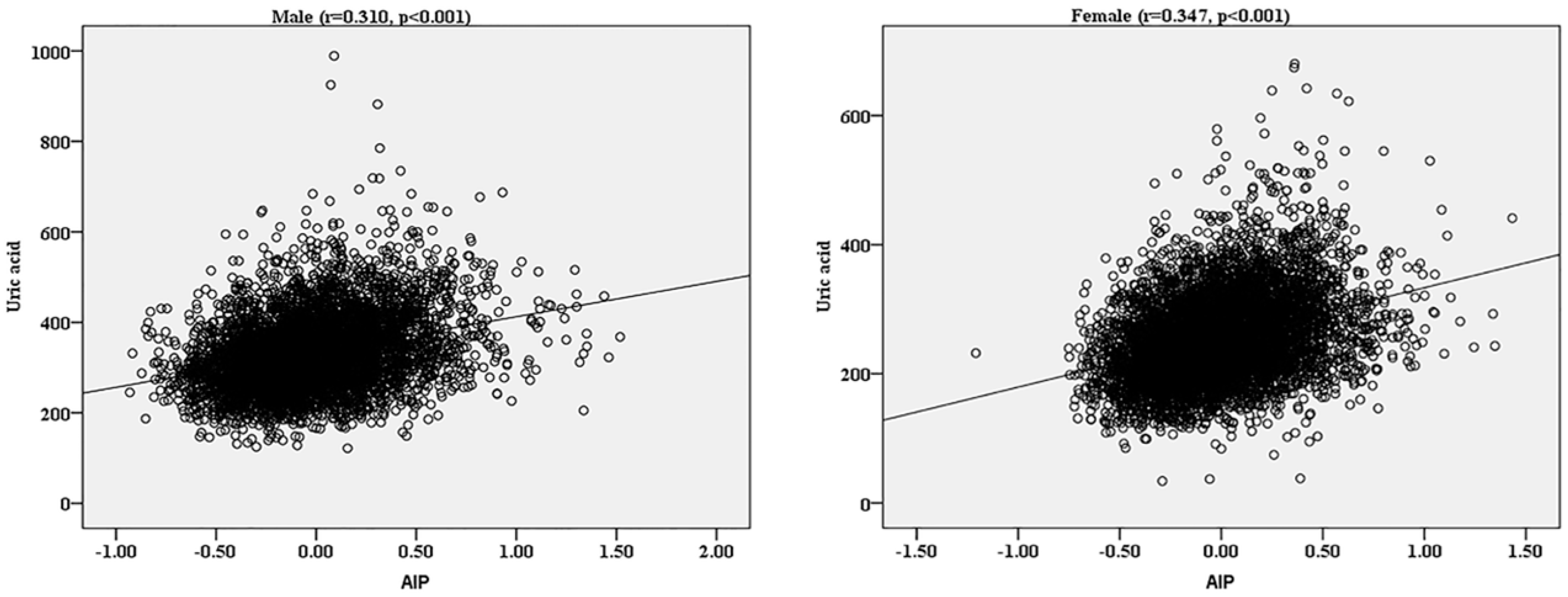
| AIP | −0.009 ± 0.32 | −0.03 ± 0.31 | 0.20 ± 0.33 | <0.001 |
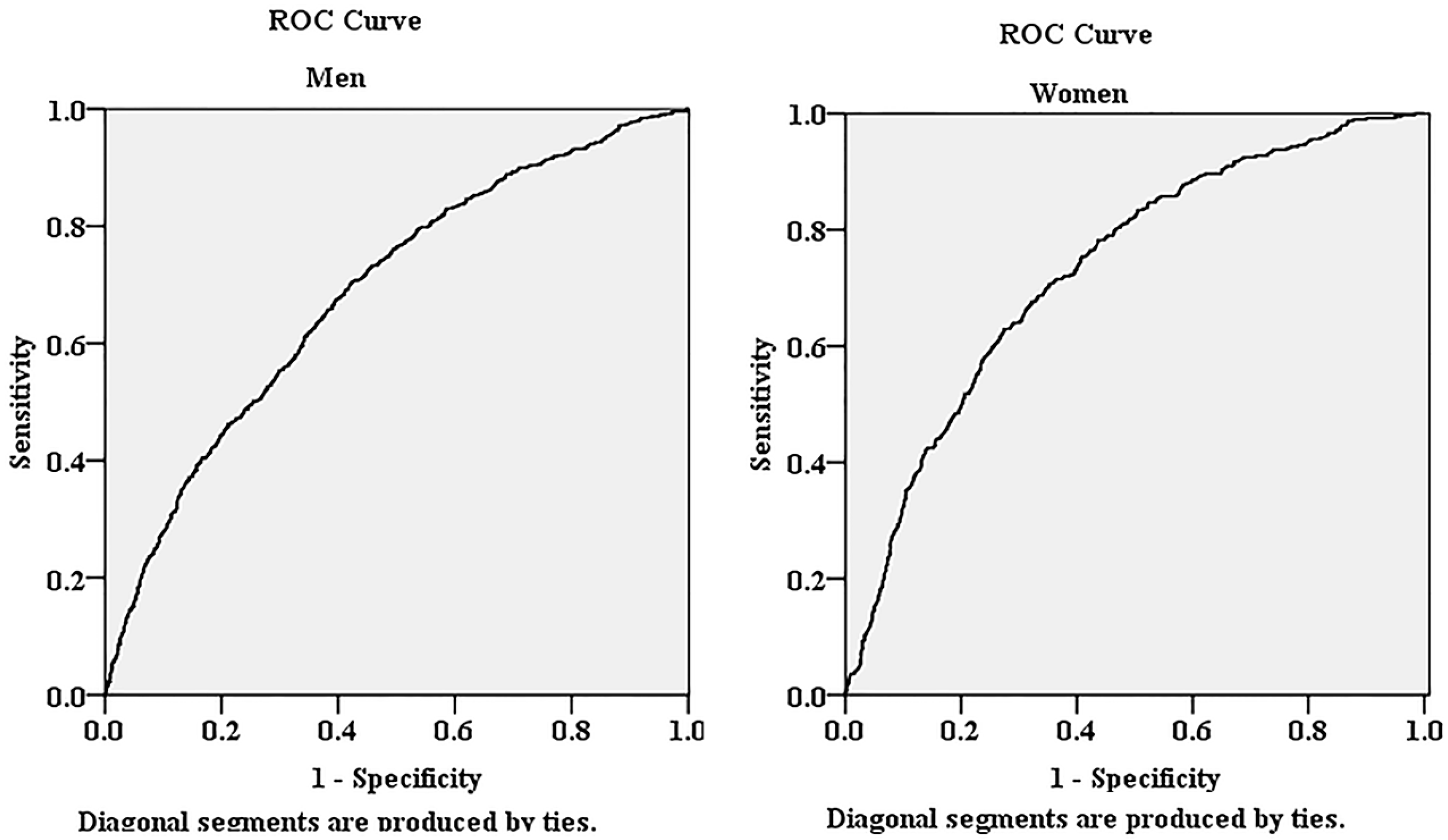
| Men | Odds Ratios | 95% Confidence Intervals | p Value |
| 1 (Referenced Group) | 1 | - | - |
| 2 | 1.315 | 0.989, 1.748 | 0.059 |
| 3 | 2.164 | 1.782, 2.628 | <0.001 |
| Women | Odds Ratios | 95% Confidence Intervals | p Value |
| 1 (Referenced Group) | 1 | - | - |
| 2 | 1.849 | 1.305, 2.620 | <0.001 |
| 3 | 2.96 | 2.311, 3.792 | <0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Li, Y.; Guo, X.; Guo, L.; Sun, Y. Atherogenic Index of Plasma Predicts Hyperuricemia in Rural Population: A Cross-Sectional Study from Northeast China. Int. J. Environ. Res. Public Health 2016, 13, 879. https://doi.org/10.3390/ijerph13090879
Chang Y, Li Y, Guo X, Guo L, Sun Y. Atherogenic Index of Plasma Predicts Hyperuricemia in Rural Population: A Cross-Sectional Study from Northeast China. International Journal of Environmental Research and Public Health. 2016; 13(9):879. https://doi.org/10.3390/ijerph13090879
Chicago/Turabian StyleChang, Ye, Yuan Li, Xiaofan Guo, Liang Guo, and Yingxian Sun. 2016. "Atherogenic Index of Plasma Predicts Hyperuricemia in Rural Population: A Cross-Sectional Study from Northeast China" International Journal of Environmental Research and Public Health 13, no. 9: 879. https://doi.org/10.3390/ijerph13090879





