Cultural and Molecular Evidence of Legionella spp. Colonization in Dental Unit Waterlines: Which Is the Best Method for Risk Assessment?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Water from DUWLs
2.2. Sampling Tap Water
2.3. Quantification of Legionella Using the Culture Method
2.4. Quantification of Viable Legionella Using PMA-qPCR
2.5. Quantification of Waterborne Bacteria
2.6. Statistical Analysis
3. Results
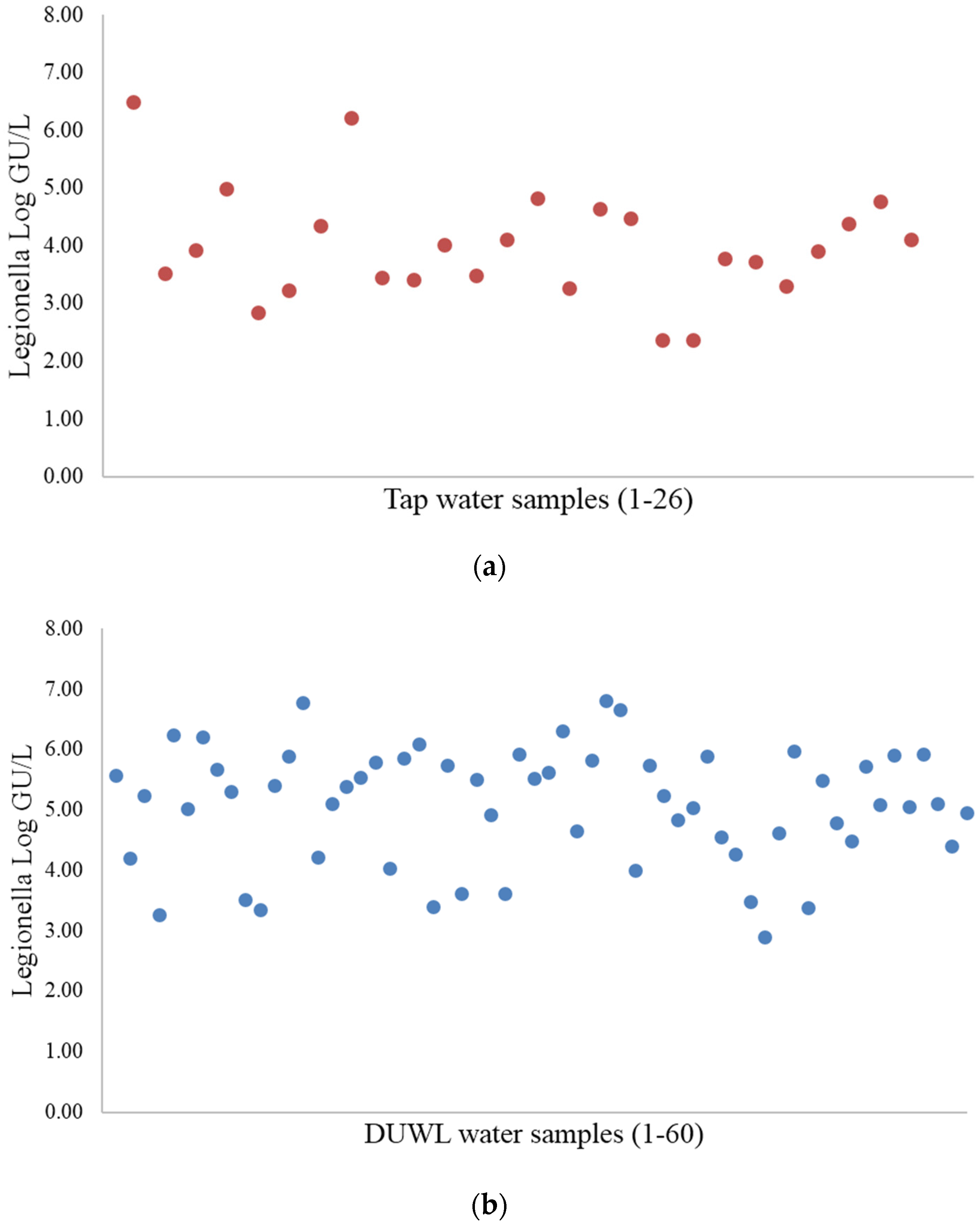
3.1. Legionella Quantification Using Culture and PMA-qPCR Methods
| Type of Sample | Culture Method | PMA-qPCR | ||
|---|---|---|---|---|
| Positive n. | Negative n. | Positive n. | Negative n. | |
| Tap water | 2 | 24 | 26 | 0 |
| DUWL output | 4 | 56 | 60 | 0 |
| Total No. (%) | 6 (7%) | 80 (93%) | 86 (100%) | (0%) |
| Sample | Legionella PMA-qPCR | Legionella Culture | TVCs 22 °C | TVCs 37 °C |
|---|---|---|---|---|
| (GU/L) | (CFU/L) | CFU/mL | CFU/mL | |
| DUWL output water | 6.7 × 104 | 4.0 × 102 | 3.0 × 103 | 4.1 × 102 |
| DUWL output water | 3.5 × 104 | 1.0 × 102 | 1.5 × 102 | 2.6 × 102 |
| DUWL output water | 1.1 × 105 | 1.2 × 103 | 1.4 × 103 | 1.5 × 102 |
| DUWL output water | 8.3 × 105 | 9.0 × 102 | 6.5 × 102 | 70 |
| Tap water | 4.2 × 104 | 8.0 × 102 | 47 | 12 |
| Tap water | 5.2 × 103 | 4.0 × 102 | 5 | 1 |
3.2. Waterborne Bacteria
| Type of Sample | TVCs | |
|---|---|---|
| 22 °C | 37 °C | |
| Tap water (geometric mean CFU/L ± SD) | 1.7 × 102 ± 5.8 × 102 | 1.3 × 103 ± 5.8 × 102 |
| Dental unit (geometric mean CFU/L ± SD) | 1.5 × 103 ± 1.4 × 103 | 1.3 × 103 ± 2.0 × 103 |
| TVCs | DUWL Water Samples (n) | Legionella-Positive Cultures (n) | Tap Water Samples (n) | Legionella-Positive Cultures (n) |
|---|---|---|---|---|
| Drinking water threshold * | 3 | 0 | 20 | 2 |
| 102–3 × 103 CFU/mL | 31 | 4 | 5 | 0 |
| >3 × 103 CFU/mL | 26 | 0 | 1 | 0 |
| Total | 60 | 4 | 26 | 2 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Atlas, R.M.; Williams, J.F.; Huntington, M.K. Legionella contamination of dental-unit waters. Appl. Environ. Microbiol. 1995, 61, 1208–1213. [Google Scholar] [PubMed]
- Barbeau, J.; Gauthier, C.; Payment, P. Biofilms, infectious agents, and dental unit waterlines: A review. Can. J. Microbiol. 1998, 44, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.T.; Bradshaw, D.J.; Finney, M.; Fulford, M.R.; Frandsen, E.; ØStergaard, E.; ten Cate, J.M.; Moorer, W.R.; Schel, A.J.; Mavridou, A.; et al. Microbiological evaluation of dental unit water systems in general dental practice in Europe. Eur. J. Oral Sci. 2004, 112, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Dutil, S.; Veillette, M.; Mériaux, A.; Lazure, L.; Barbeau, J.; Duchaine, C. Aerosolization of Mycobacteria and legionellae during dental treatment: Low exposure despite dental unit contamination. Environ. Microbiol. 2007, 9, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Bristela, M.; Skolka, A.; Schmid-Schwap, M.; Piehslinger, E.; Indra, A.; Wewalka, G.; Stauffer, F. Testing for aerobic heterotrophic bacteria allows no prediction of contamination with potentially pathogenic bacteria in the output water of dental chair units. GMS Krankenhaushyg. Interdiszip. 2012, 7. [Google Scholar] [CrossRef]
- Arvand, M.; Hack, A. Microbial contamination of dental unit waterlines in dental practices in Hesse, Germany: A cross-sectional study. Eur. J. Microbiol. Immunol. 2013, 3, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Vincent-Houdek, M.; Muytjens, H.L.; Bongaerts, G.P.; van Ketel, R.J. Legionella monitoring: A continuing story of nosocomial infection prevention. J. Hosp. Infect. 1993, 25, 117–124. [Google Scholar] [CrossRef]
- Kool, J.L.; Bergmire-Sweat, D.; Butler, J.C.; Brown, E.W.; Peabody, D.J.; Massi, D.S.; Carpenter, J.C.; Pruckler, J.M.; Benson, R.F.; Fields, B.S. Hospital characteristics associated with colonization of water systems by Legionella and risk of nosocomial legionnaires’ disease: A cohort study of 15 hospitals. Infect. Control Hosp. Epidemiol. 1999, 20, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Wellinghausen, N.; Frost, C.; Marre, R. Detection of legionellae in hospital water samples by quantitative real-time LightCycler PCR. Appl. Environ. Microbiol. 2001, 67, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.L.; Fontana, S.; Pinci, F.; Fiumana, E.; Pedna, M.F.; Farolfi, P.; Sabattini, M.A.B.; Scaturro, M. Pneumonia associated with a dental unit waterline. Lancet 2012, 379. [Google Scholar] [CrossRef]
- Pankhurst, C.L. Risk assessment of dental unit waterline contamination. Prim. Dent. Care 2003, 10, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Isituto Superiore di Sanità. Rapporto Annuale Sulla Legionellosi in Italia Nel 2013; Isituto Superiore di Sanità: Roma, Italy, 2014; pp. 3–9. (In Italian) [Google Scholar]
- Ministero della Salute. Linee Guida Per La Prevenzione ed Il Controllo Della Legionellosi; Ministero della Salute: Roma, Italy, 2015. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2362_allegato.pdf (accessed on 4 February 2016). (In Italian)
- Rowbotham, T.J. Current views on the relationships between amoebae, legionellae and man. Isr. J. Med. Sci. 1986, 22, 678–689. [Google Scholar] [PubMed]
- Berk, S.G.; Ting, R.S.; Turner, G.W.; Ashburn, R.J. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl. Environ. Microbiol. 1998, 64, 279–286. [Google Scholar] [PubMed]
- Swanson, M.S.; Hammer, B.K. Legionella pneumophila pathogesesis: A fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 2000, 54, 567–613. [Google Scholar] [CrossRef] [PubMed]
- Michel, R.; Borneff, M. The significance of amoebae and other protozoa in water conduit systems in dental units. Zentralblatt Bakteriol. Mikrobiol. Hyg. Ser. B 1989, 187, 312–323. [Google Scholar]
- Villari, P.; Motti, E.; Farullo, C.; Torre, I. Comparison of conventional culture and PCR methods for the detection of Legionella pneumophila in water. Lett. Appl. Microbiol. 1998, 27, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.L.; Koh, B.B.; Tay, L.; Heng, B.H. Comparison of polymerase chain reaction and conventional culture for the detection of legionellae in cooling tower waters in Singapore. Lett. Appl. Microbiol. 1997, 24, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.N.; Maas, E.; Martin, T.; Harte, D.; Grubner, M.; Margolin, T. Investigation of the prevalence of Legionella species in domestic hot water systems. N. Z. Med. J. 2000, 113, 218–220. [Google Scholar] [PubMed]
- Ditommaso, S.; Ricciardi, E.; Giacomuzzi, M.; Arauco Rivera, S.R.; Ceccarelli, A.; Zotti, C.M. Overestimation of the Legionella spp. load in environmental samples by quantitative real-time PCR: Pretreatment with propidium monoazide as a tool for the assessment of an association between Legionella concentration and sanitary risk. Diagn. Microbiol. Infect. Dis. 2014, 80, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Ditommaso, S.; Gentile, M.; Giacomuzzi, M.; Zotti, C.M. Recovery of Legionella species from water samples using an internal method based on ISO 11731: Suggestions for revision and implementation. Diagn. Microbiol. Infect. Dis. 2011, 70, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ditommaso, S.; Ricciardi, E.; Giacomuzzi, M.; Arauco Rivera, S.R.; Zotti, C.M. Legionella in water samples: How can you interpret the results obtained by quantitative PCR? Mol. Cell. Probes 2015, 29, 7–12. [Google Scholar] [CrossRef] [PubMed]
- International Standards Organization (ISO). Water Quality—Enumeration of Culturable Micro-organisms—Colony Count by Inoculation in A Nutrient Agar Culture Medium; ISO 6222; International Standards Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; American Public Health Association; American Water Works Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Wadowsky, R.M.; Wolford, R.; McNamara, A.M.; Yee, R.B. Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl. Environ. Microbiol. 1985, 49, 1197–1205. [Google Scholar] [PubMed]
- Walker, J.T.; Bradshaw, D.J.; Bennett, A.M.; Fulford, M.R.; Martin, M.V.; Marsh, P.D. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl. Environ. Microbiol. 2000, 66, 3363–3367. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.E. Waterborne pathogens and dental waterlines. Dent. Clin. N. Am. 2003, 47, 545–557. [Google Scholar] [CrossRef]
- Pankhurst, C.L.; Coulter, W.; Philpott-Howard, J.J.; Harrison, T.; Warburton, F.; Platt, S.; Surman, S.; Challacombe, S. Prevalence of Legionella waterline contamination and Legionella pneumophila antibodies in general dental practitioners in London and rural northern Ireland. Brit. Dent. J. 2003, 195, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Ma’ayeh, S.Y.; Al-Hiyasat, A.S.; Hindiyeh, M.Y.; Khader, Y.S. Legionella pneumophila contamination of a dental unit water line system in a dental teaching centre. Int. J. Dent. Hyg. 2008, 6, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, J.; Tanguay, R.; Faucher, E.; Avezard, C.; Trudel, L.; Côté, L.; Prévost, A.P. Multiparametric analysis of waterline contamination in dental units. Appl. Environ. Microbiol. 1996, 62, 3954–3959. [Google Scholar] [PubMed]
- Williams, H.N.; Paszko-Kolva, C.; Shahamat, M.; Palmer, C.; Pettis, C.; Kelley, J. Molecular techniques reveal high prevalence of Legionella in dental units. J. Am. Dent. Assoc. 1996, 127, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Dutil, S.; Tessier, S.; Veillette, M.; Laflamme, C.; Mériaux, A.; Leduc, A.; Barbeau, J.; Duchaine, C. Detection of Legionella spp. by fluorescent in situ hybridization in dental unit waterlines. J. Appl. Microbiol. 2006, 100, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Noce, L.; di Giovanni, D.; Putnins, E.E. An evaluation of sampling and laboratory procedures for determination of heterotrophic plate counts in dental unit waterlines. J. Can. Dent. Assoc. 2000, 66, 262. [Google Scholar] [PubMed]
- Szymanska, J. Biofilm and dental unit waterlines. Ann. Agric. Environ. Med. 2003, 10, 151–157. [Google Scholar] [PubMed]
- Leoni, E.; Dallolio, L.; Stagni, F.; Sanna, T.; D’Alessandro, G.; Piana, G. Impact of a risk management plan on Legionella contamination of dental unit water. Int. J. Environ. Res. Public. Health 2015, 12, 2344–2358. [Google Scholar] [CrossRef] [PubMed]
- Toze, S.; Sly, L.I.; Macrae, I.C.; Fuerst, J.A. Inhibition of growth of Legionella species by heterotrophic plate count bacteria isolated from chlorinated drinking water. Curr. Microbiol. 1990, 21, 139–143. [Google Scholar] [CrossRef]
- Byrne, B.; Swanson, M.S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 1998, 66, 3029–3034. [Google Scholar] [PubMed]
- Ohata, K.; Sugiyama, K.; Suzuki, M.; Shimogawara, R.; Izumiyama, S.; Yagita, K.; Endo, T. Growth of Legionella in nonsterilized, naturally contaminated bathing water in a system that circulates the water. In Legionella; ASM Press: Washington, DC, USA, 2006. [Google Scholar]
- Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. Available online: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5217a1.htm (accessed on 4 February 2016).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditommaso, S.; Giacomuzzi, M.; Ricciardi, E.; Zotti, C.M. Cultural and Molecular Evidence of Legionella spp. Colonization in Dental Unit Waterlines: Which Is the Best Method for Risk Assessment? Int. J. Environ. Res. Public Health 2016, 13, 211. https://doi.org/10.3390/ijerph13020211
Ditommaso S, Giacomuzzi M, Ricciardi E, Zotti CM. Cultural and Molecular Evidence of Legionella spp. Colonization in Dental Unit Waterlines: Which Is the Best Method for Risk Assessment? International Journal of Environmental Research and Public Health. 2016; 13(2):211. https://doi.org/10.3390/ijerph13020211
Chicago/Turabian StyleDitommaso, Savina, Monica Giacomuzzi, Elisa Ricciardi, and Carla M. Zotti. 2016. "Cultural and Molecular Evidence of Legionella spp. Colonization in Dental Unit Waterlines: Which Is the Best Method for Risk Assessment?" International Journal of Environmental Research and Public Health 13, no. 2: 211. https://doi.org/10.3390/ijerph13020211





