Evaluation of the Survivability of Microorganisms Deposited on Filtering Respiratory Protective Devices under Varying Conditions of Humidity
Abstract
:1. Introduction
2. Experimental Section
2.1. Tested Respirators and Filter Materials
2.2. Experimental Design and Test Conditions
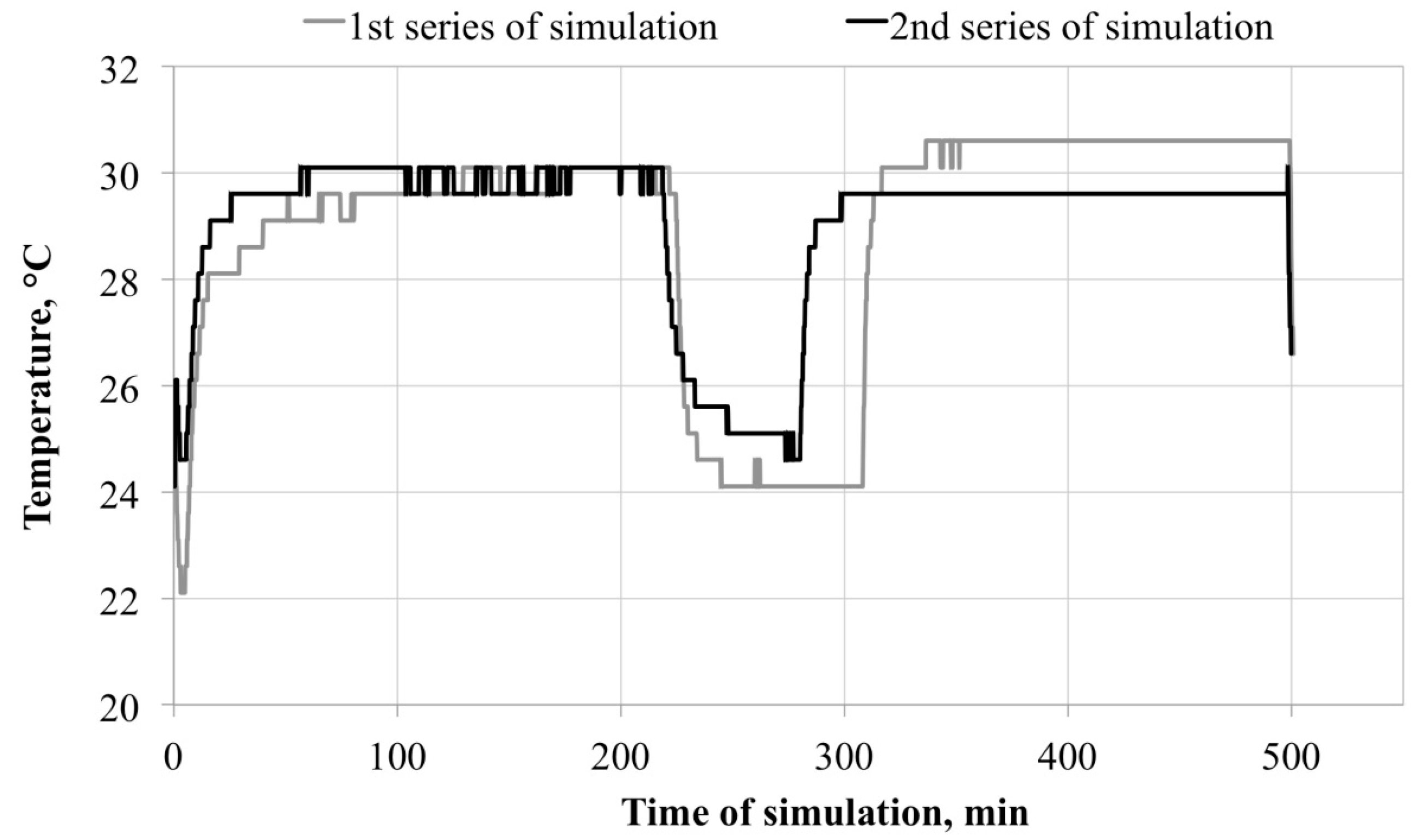
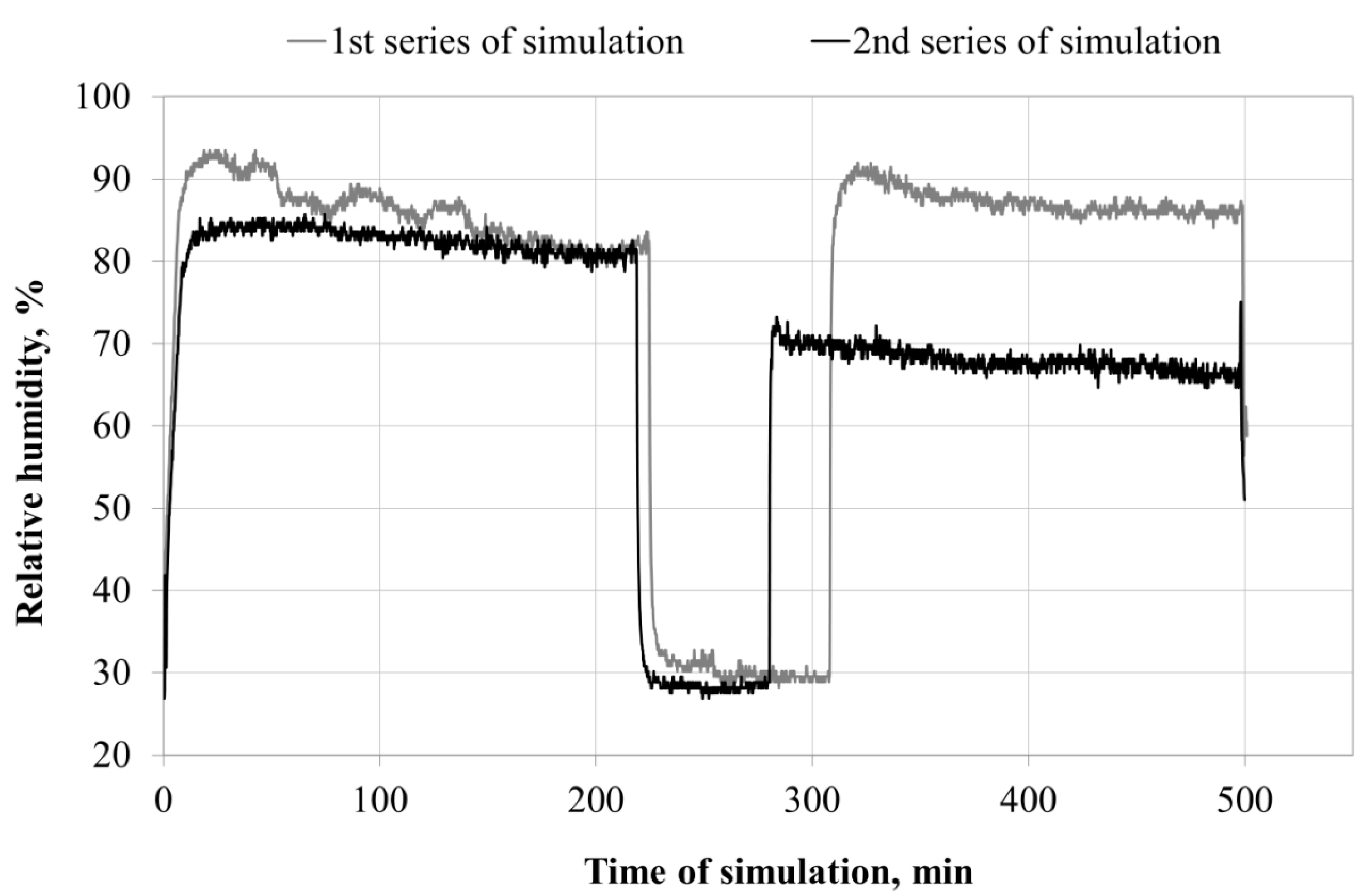
Microclimate inside the FFRs
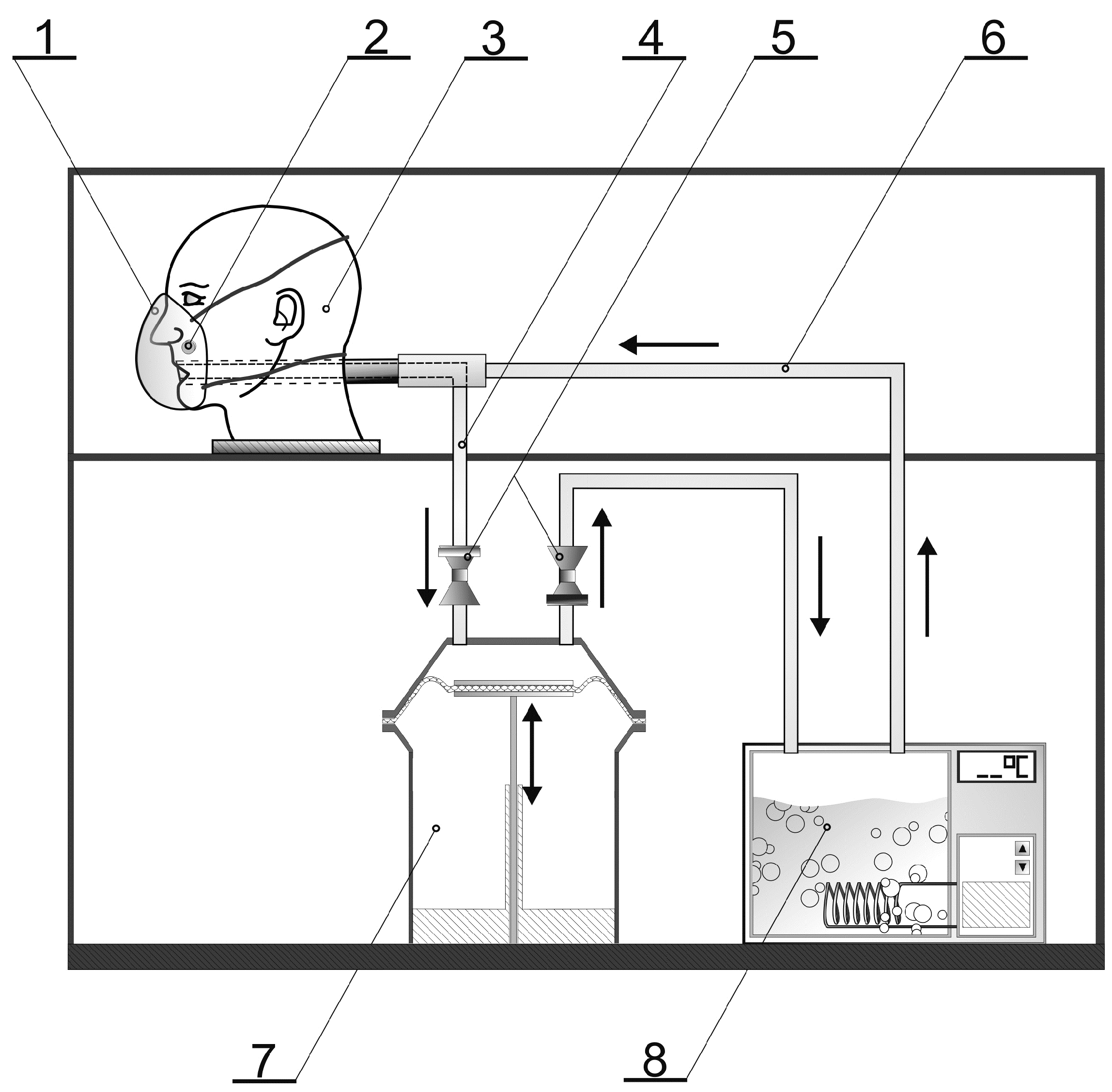
2.3. Microorganisms
2.4. Survivability of Microorganisms on Filter Materials
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microclimate inside the FFRs
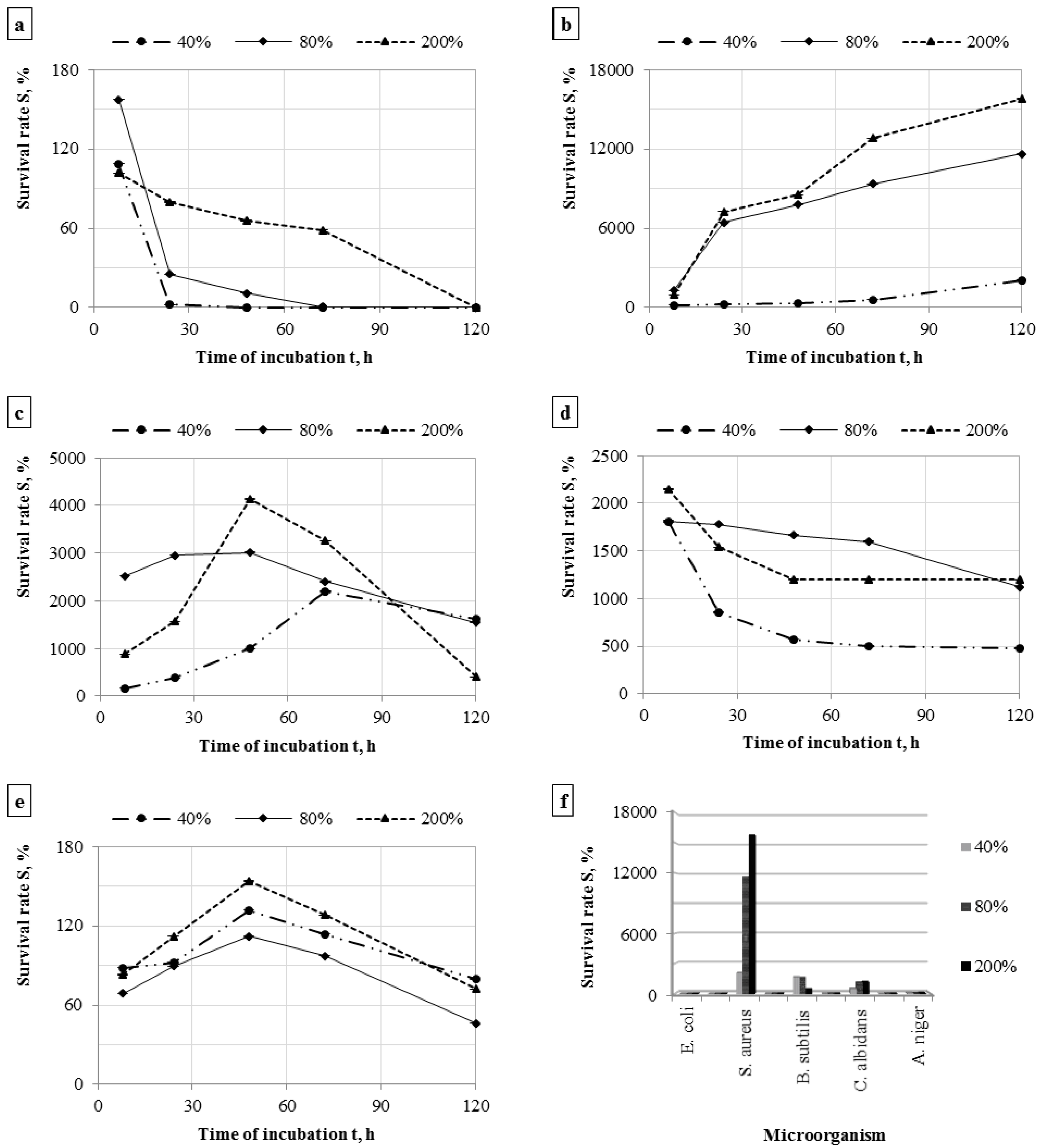
3.2. Survivability of Microorganisms on Filter Materials
| Microorganism | Mass Humidity, % | The Number of Cells on the Nonwoven Fabric after Incubation, cfu/sample (n = 6) | |||||
|---|---|---|---|---|---|---|---|
| 0 h | 8 h | 24 h | 48 h | 72 h | 120 h | ||
| Escherichia coli | 40 | M: 8.75 × 105 | M: 9.53 × 105 | M: 1.79 × 104 * | M: 9.18 × 101 * | M: 2.07 × 101 * | M: 9.83 × 100 * |
| SD: 1.42 × 105 | SD: 1.14 × 105 | SD: 4.05 × 103 | SD: 3.82 × 101 | SD: 1.18 × 101 | SD: 6.91 × 100 | ||
| 80 | M: 1.76 × 106 | M: 2.77 × 106 * | M: 4.45 × 105 * | M: 1.93 × 105 * | M: 1.11 × 104 * | M: 2.75 × 102 * | |
| SD: 7.34 × 105 | SD: 3.95 × 105 | SD: 1.92 × 105 | SD: 1.72 × 104 | SD: 6.45 × 103 | SD: 1.08 × 102 | ||
| 200 | M: 2.77 × 106 | M: 2.82 × 106 | M: 2.21 × 106 * | M: 1.82 × 106 * | M: 1.62 × 106 * | M: 2.50 × 103 * | |
| SD: 2.73 × 105 | SD: 9.81 × 105 | SD: 3.93 × 105 | SD: 6.17 × 105 | SD: 1.83 × 105 | SD: 7.90 × 102 | ||
| Staphylococcus aureus | 40 | M: 1.93 × 104 * | M: 3.23 × 104 * | M: 4.78 × 104 * | M: 5.95 × 104 * | M: 1.14 × 105 | M: 4.02 × 105 * |
| SD: 8.91 × 103 | SD: 4.08 × 103 | SD: 1.87 × 104 | SD: 2.08 × 104 | SD: 2.08 × 105 | SD: 2.19 × 105 | ||
| 80 | M: 1.36 × 104 | M: 1.74 × 105 * | M: 8.75 × 105 * | M: 1.06 × 106 * | M: 1.27 × 106 * | M: 1.58 × 106 * | |
| SD: 3.29 × 103 | SD: 2.99 × 104 | SD: 1.93 × 105 | SD: 2.41 × 105 | SD: 2.43 × 105 | SD: 1.46 × 105 | ||
| 200 | M: 1.57 × 104 | M: 1.51 × 105 * | M: 1.14 × 106 * | M: 1.34 × 106 * | M: 2.01 × 106 * | M: 2.48 × 106 * | |
| SD: 1.65 × 104 | SD: 3.69 × 104 | SD: 1.61 × 105 | SD: 1.70 × 105 | SD: 3.22 × 105 | SD: 4.60 × 105 | ||
| Bacillus subtilis | 40 | M: 4.37 × 103 | M: 6.97 × 103 * | M: 1.67 × 104 * | M: 4.38 × 104 * | M: 9.58 × 104 * | M: 7.13 × 104 * |
| SD: 1.50 × 103 | SD: 1.10 × 103 | SD: 8.16 × 103 | SD: 1.82 × 104 | SD: 2.64 × 104 | SD: 1.45 × 104 | ||
| 80 | M: 6.70 × 103 | M: 1.69 × 105 * | M: 1.98 × 105 | M: 2.02 × 105 * | M: 1.61 × 105 * | M: 1.04 × 105 * | |
| SD: 2.74 × 103 | SD: 4.58 × 104 | SD: 2.35 × 105 | SD: 7.65 × 104 | SD: 1.46 × 105 | SD: 1.81 × 104 | ||
| 200 | M: 1.30 × 104 | M: 1.15 × 105 * | M: 2.04 × 105 * | M: 5.37 × 105 * | M: 4.24 × 105 * | M: 5.21 × 104 * | |
| SD: 5.93 × 103 | SD: 1.70 × 104 | SD: 6.29 × 104 | SD: 2.99 × 105 | SD: 1.47 × 105 | SD: 3.72 × 104 | ||
| Candida albidans | 40 | M: 3.10 × 103 | M: 5.61 × 104 | M: 2.65 × 104 * | M: 1.78 × 104 * | M: 1.57 × 104 * | M: 1.50 × 104 * |
| SD: 1.33 × 103 | SD: 6.20 × 104 | SD: 5.89 × 103 | SD: 3.29 × 103 | SD: 1.66 × 103 | SD: 5.42 × 103 | ||
| 80 | M: 2.78 × 103 | M: 5.03 × 104 * | M: 4.94 × 104 * | M: 4.63 × 104 | M: 4.44 × 104 * | M: 3.12 × 104 * | |
| SD: 1.47 × 103 | SD: 8.82 × 103 | SD: 1.60 × 104 | SD: 5.83 × 104 | SD: 1.32 × 104 | SD: 1.35 × 104 | ||
| 200 | M: 2.48 × 103 | M: 5.32 × 104 * | M: 3.81 × 104 * | M: 2.97 × 104 * | M: 2.96 × 104 * | M: 2.96 × 104 * | |
| SD: 8.68 × 102 | SD: 1.97 × 104 | SD: 1.52 × 104 | SD: 9.94 × 103 | SD: 2.21 × 104 | SD: 1.84 × 104 | ||
| Aspergillus niger | 40 | M: 1.38 × 103 | M: 1.22 × 103 | M: 1.27 × 103 | M: 1.82 × 103 | M: 1.57 × 103 | M: 1.10 × 103 |
| SD: 5.46 × 102 | SD: 8.82 × 102 | SD: 8.62 × 102 | SD: 7.11 × 102 | SD: 5.66 × 102 | SD: 5.21 × 102 | ||
| 80 | M: 2.33 × 103 | M: 1.60 × 103 | M: 2.08 × 103 | M: 2.61 × 103 | M: 2.27 × 103 | M: 1.08 × 103 * | |
| SD: 1.06 × 103 | SD: 5.69 × 102 | SD: 7.55 × 102 | SD: 1.78 × 103 | SD: 9.84 × 102 | SD: 2.14 × 102 | ||
| 200 | M: 2.07 × 103 | M: 1.72 × 103 | M: 2.32 × 103 | M: 3.19 × 103 | M: 2.65 × 103 | M: 1.49 × 103 | |
| SD: 1.02 × 103 | SD: 3.92 × 102 | SD: 7.55 × 102 | SD: 1.90 × 103 | SD: 4.64 × 102 | SD: 5.04 × 102 | ||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lacey, J.; Dutkiewicz, J. Bioaerosols and occupational lung disease. J. Aerosol. Sci. 1994, 25, 1371–1404. [Google Scholar] [CrossRef]
- Corrao, C.R.N.; Mazzotta, A.; la Torre, G.; de Giusti, M. Biological risk and occupational health. Ind. Health 2012, 50, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Reanprayoon, P.; Yoonaiwong, W. Airborne concentrations of bacteria and fungi in Thailand border market. Aerobiologia 2012, 28, 49–60. [Google Scholar] [CrossRef]
- Sudharsanam, S.; Swaminathan, S.; Ramalingam, A.; Thangavel, G.; Annamalai, R.; Steinberg, R.; Balakrishanan, K.; Srikanth, P. Characterization of indoor bioaerosols from a hospital ward in a tropical setting. Afr. Health Sci. 2012, 12, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Krysińska-Traczyk, E.; Pande, B.N.; Skórska, C.; Sitkowska, J.; Prażmo, Z.; Cholewa, G.; Dutkiewicz, J. Exposure of Indian agricultural workers to airborne microorganisms, dust and endotoxin during handling of various plant products. Ann. Agric. Environ. Med. 2005, 12, 269–275. [Google Scholar]
- Coggins, M.A.; Hogan, V.J.; Kelly, M.; Fleming, G.T.A.; Roberts, N.; Tynan, T.; Thorne, P.S. Workplace exposure to bioaerosols in podiatry clinics. Ann. Occup. Hyg. 2012, 56, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, J. Dental bioaerosol as an occupational hazard in a dentist’s workplace. Ann. Agric. Environ. Med. 2007, 14, 203–207. [Google Scholar] [PubMed]
- Hansen, V.M.; Meyling, N.V.; Winding, A.; Eilenberg, J.; Madsen, A.M. Factors affecting vegetable growers’ exposure to fungal bioaerosols and airborne dust. Ann. Occup. Hyg. 2012, 56, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Ernst, S.; Lotz, G.; Linsel, G.; Jäckel, U. Microbial exposure and respiratory dysfunction in poultry hatchery workers. Environ. Sci. Process. Impacts 2013, 15, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Faísca, V.M.; Dias, H.; Clérigo, A.; Carolino, E.; Viegasa, C. Occupational exposure to poultry dust and effects on the respiratory system in workers. J. Toxicol. Environ. Health 2013, 76, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Jerez, S.B.; Cheng, Y.; Bray, J. Exposure of workers to dust and bioaerosol on a poultry farm. J. Appl. Poult. Res. 2014, 23, 7–14. [Google Scholar] [CrossRef]
- Lawniczek-Walczyk, A.; Gorny, R.L.; Golofit-Szymczak, M.; Niesler, A.; Wlazlo, A. Occupational exposure to airborne microorganisms, endotoxins and beta-glucans in poultry houses at different stages of the production cycle. Ann. Agric. Environ. Med. 2013, 20, 259–268. [Google Scholar] [PubMed]
- Sowiak, M.; Bródka, K.; Buczyńska, A.; Cyprowski, M.; Kozajda, A.; Sobala, W.; Szadkowska-Stańczyk, I. An assessment of potential exposure to bioaerosols among swine farm workers with particular reference to airborne microorganisms in the respirable fraction under various breeding conditions. Aerobiologia 2012, 28, 121–133. [Google Scholar] [CrossRef]
- Lee, S.A.; Liao, C.H. Size-selective assessment of agricultural workers’ personal exposure to airborne fungi and fungal fragments. Sci. Total Environ. 2014, 466, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Rusca, S.; Charrière, N.; Droz, P.O.; Oppliger, A. Effects of bioaerosol exposure on work-related symptoms among Swiss sawmill workers. Int. Arch. Occup. Environ. Health 2008, 81, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Alwis, K.U.; Mandryk, J.; Hocking, A.D. Exposure to biohazards in wood dust: Bacteria, fungi, endotoxins, and (1 3)-beta-d-glucans. Appl. Occup. Environ. Hyg. 1999, 14, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Gutarowska, B.; Skóra, J.; Stępień, Ł.; Szponar, B.; Otlewska, A.; Pielech-Przybylska, K. Assessment of microbial contamination within working environments of different types of composting plants. J. Air Waste Manage. Assoc. 2015, 65, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, O.; Godon, J.J.; Milferstedt, K.; Bacheley, H.; Steyer, J.P.; Wéry, N. A new combination of microbial indicators for monitoring composting bioaerosols. Atmos. Environ. 2012, 61, 428–433. [Google Scholar] [CrossRef]
- Wéry, N. Bioaerosols from composting facilities—A review. Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef]
- Hagemeyer, O.; Bünger, J.; van Kampen, V.; Raulf-Heimsoth, M.; Drath, C.; Merget, R.; Brüning, T.H.; Broding, H.C. Occupational allergic respiratory diseases in garbage workers: Relevance of molds and actinomycetes. Adv. Exp. Med. Biol. 2013, 788, 313–320. [Google Scholar] [PubMed]
- Carducci, A.; Federigi, I.; Verani, M. Virus occupational exposure in solid waste processing facilities. Ann. Occup. Hyg. 2013, 57, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Simon, X.; Duquenne, P. Assessment of workers’ exposure to bioaerosols in a French cheese factory. Ann. Occup. Hyg. 2014, 58, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Kullman, G.J.; Thorne, P.S.; Waldron, P.F.; Marx, J.J.; Ault, B.; Lewis, D.M.; Siegel, P.D.; Olenchock, S.A.; Merchant, J.A. Organic dust exposures from work in dairy barns. Am. Ind. Hyg. Assoc. J. 1998, 59, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, J.; Cisak, E.; Sroka, J.; Wójcik-Fatla, A.; Zając, V. Biological agents as occupational hazards-selected issues. Ann. Agric. Environ. Med. 2011, 18, 286–293. [Google Scholar] [PubMed]
- Peters, S.; Kromhout, H.; Olsson, A.C.; Wichmann, H.-E.; Bruske, I.; Consonni, D.; Brüske, I.; Consonni, D.; Landi, M.T.; Caporaso, N.; et al. Occupational exposure to organic dust increases lung cancer risk in the general population. Thorax 2012, 67, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.M.; Shaffer, R.E. Considerations for recommending extended use and limited reuse of filtering facepiece respirators in health care settings. J. Occup. Environ. Hyg. 2014, 11, D115–D128. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Occupational Safety and Health (NIOSH). NIOSH Guide to the Selection and Use of Particulate Respirators Certified Under 42 CFR 84 (96-101). Available online: http://www.cdc.gov/niosh/docs/96-101/ (accessed on 1 September 2015).
- Harber, P.; Barnhart, S.; Boehlecke, B.A.; Beckett, W.S.; Gerrity, T.; McDiarmid, M.A.; Nardbell, E.; Repsher, L.; Brousseau, L.; Hodous, T.K.; et al. Respiratory protection guidelines. Am. J. Respir. Crit. Care Med. 1996, 154, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Guidance for Industry and FDA Staff: Surgical Masks—Premarket Notification [510(k)] Submissions. Available online: http://www.fda.gov/OHRMS/DOCKETS/98fr/03d-0137-gdl0002.pdf (accessed on 1 September 2015).
- Brosseau, L.M.; McCullough, N.V.; Vesley, D. Bacterial survival on respirator filters and surgical masks. J. Am. Biol. Saf. Assoc. 1997, 2, 32–43. [Google Scholar]
- Reponen, T.A.; Wang, Z.; Willeke, K.; Grinshpun, S.A. Survival of mycobacteria on N95 personal respirators on JSTOR. Infect. Control Hosp. Epidemiol. 1999, 20, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Reponen, T.; Willke, K.; Grinshpun, S.A. Survival of bacteria on respirator filters. Aerosol Sci. Technol. 1999, 30, 300–308. [Google Scholar] [CrossRef]
- Pasanen, A.-L.; Nikulin, M.; Berg, S.; Hintikka, E.-L. Stachybotrys atra corda may produce mycotoxins in respirator filters in humid environments. Am. Ind. Hyg. Assoc. J. 1994, 55, 62–65. [Google Scholar] [CrossRef]
- Johnson, B.; Winters, D.R.; Shreeve, T.R.; Coffey, C.C. Respirator filter reuse test using the laboratory simulant mycobacterium tuberculosis (H37RA strain). J. Am. Biol. Saf. Assoc. 1998, 3, 105–116. [Google Scholar]
- Maus, R.; Goppelsröder, A.; Umhauer, H. Viability of bacteria in unused air filter media. Atmos. Environ. 1997, 31, 2305–2310. [Google Scholar] [CrossRef]
- Maus, R.; Goppelsröder, A.; Umhauer, H. Survival of bacterial and mold spores in air filter media. Atmos. Environ. 2001, 35, 105–113. [Google Scholar] [CrossRef]
- Jankowska, E.; Reponen, T.; Willeke, K.; Grinshpun, S.A.; Choi, K.J. Collection of fungal spores on air filters and spore reentrainment from filters into air. J. Aerosol Sci. 2000, 31, 969–978. [Google Scholar] [CrossRef]
- Brosseau, L.M.; McCullough, N.V.; Vesley, D. Mycobacterial aerosol collection efficiency of respirator and surgical mask filters under varying conditions of flow and humidity. Appl. Occup. Environ. Hyg. 1997, 12, 435–445. [Google Scholar] [CrossRef]
- Gutarowska, B.; Michalski, A. Microbial Degradation of Woven Fabrics and Protection against Biodegradation. Available online: http://www.intechopen.com/books/woven-fabrics/microbial-degradation-of-the-woven-fabrics-and-protection-against-biodegradation (accessed on 1 September 2015).
- Majchrzycka, K.; Gutarowska, B.; Brochocka, A. Aspects of tests and assessment of filtering materials used for respiratory protection against bioaerosols. Part II: Sweat in the environment, microorganisms in the form of a bioaerosol. Int. J. Occup. Saf. Ergon. 2010, 16, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Respiratory Protective Devices. Filtering Half Masks to Protect against Particles. Requirements, Testing, Marking; BSI: London, UK, 2001. [Google Scholar]
- Directive 89/686/EEC—Personal Protective Equipment of 21 December 1989 on the Approximation of the Laws of the Member States Relating to Personal Protective Equipment. Available online: https://osha.europa.eu/en/legislation/directives/34 (accessed on 1 September 2015).
- Directive 2000/54/EC—Biological Agents at Work. Available online: https://osha.europa.eu/en/legislation/directives/exposure-to-biological-agents/77 (accessed on 1 September 2015).
- Dutkiewicz, J.; Śpiewak, R.; Jabłoński, L.; Szymańska, J. Biological Occupational Risk Factors. Classification, Exposed Occupational Groups, Measurement, Prevention; Ad Punctum: Lublin, Poland, 2007. [Google Scholar]
- Antibacterial Finishes on Textile Materials: Assessment of. AATCC Technical Manual, 2010. Available online: http://shanghaijifa.com/UploadFile/201104/AATCC2010%E8%8B%B1%E6%96%87%E7%89%88.pdf (accessed on 1 September 2015).
- Raynor, P.C.; Leith, D. The influence of accumulated liquid on fibrous filter performance. J. Aerosol Sci. 2000, 31, 19–34. [Google Scholar] [CrossRef]
- Walsh, D.C.; Stenhouse, J.I.T.; Scurrah, K.L.; Graef, A. Effect of solid and liquid aerosol particle loading on fibrous filter material performance. J. Aerosol. Sci. 1996, 27, 617–618. [Google Scholar] [CrossRef]
- Agranovski, I.E.; Braddock, R.D. Filtration of liquid aerosols on nonwettable fibrous filters. AIChE J. 1998, 44, 2784–2790. [Google Scholar] [CrossRef]
- Agranovski, I.E.; Braddock, R.D. Filtration of liquid aerosols on wettable fibrous filters. AIChE J. 1998, 44, 2775–2783. [Google Scholar] [CrossRef]
- Pasanen, A.L.; Keinanen, J.; Kalliokoski, P.; Martikainen, P.I.; Ruuskanen, J. Microbial growth on respirator filters from improper storage. Scand. J. Work Environ. Health 1993, 19, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. Available online: http://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html (accessed on 26 October 2015).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majchrzycka, K.; Okrasa, M.; Skóra, J.; Gutarowska, B. Evaluation of the Survivability of Microorganisms Deposited on Filtering Respiratory Protective Devices under Varying Conditions of Humidity. Int. J. Environ. Res. Public Health 2016, 13, 98. https://doi.org/10.3390/ijerph13010098
Majchrzycka K, Okrasa M, Skóra J, Gutarowska B. Evaluation of the Survivability of Microorganisms Deposited on Filtering Respiratory Protective Devices under Varying Conditions of Humidity. International Journal of Environmental Research and Public Health. 2016; 13(1):98. https://doi.org/10.3390/ijerph13010098
Chicago/Turabian StyleMajchrzycka, Katarzyna, Małgorzata Okrasa, Justyna Skóra, and Beata Gutarowska. 2016. "Evaluation of the Survivability of Microorganisms Deposited on Filtering Respiratory Protective Devices under Varying Conditions of Humidity" International Journal of Environmental Research and Public Health 13, no. 1: 98. https://doi.org/10.3390/ijerph13010098








