Glyceollin I Reverses Epithelial to Mesenchymal Transition in Letrozole Resistant Breast Cancer through ZEB1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Proliferation Assays
2.3. Colony Formation Assays
2.4. Cell Migration and Invasion Assays
2.5. Gel Electrophoresis and Western Blot Analysis
2.6. Quantitative RT-PCR
2.7. Gene Expression Superarrays
2.8. Immunofluorescence
2.9. Animal Xenograft Studies
2.10. Immunohistochemistry
2.11. Statistical Analysis
3. Results
3.1. Glyceollin I Inhibits Proliferation and Viability of Letrozole-Resistant Breast Cancer Cells
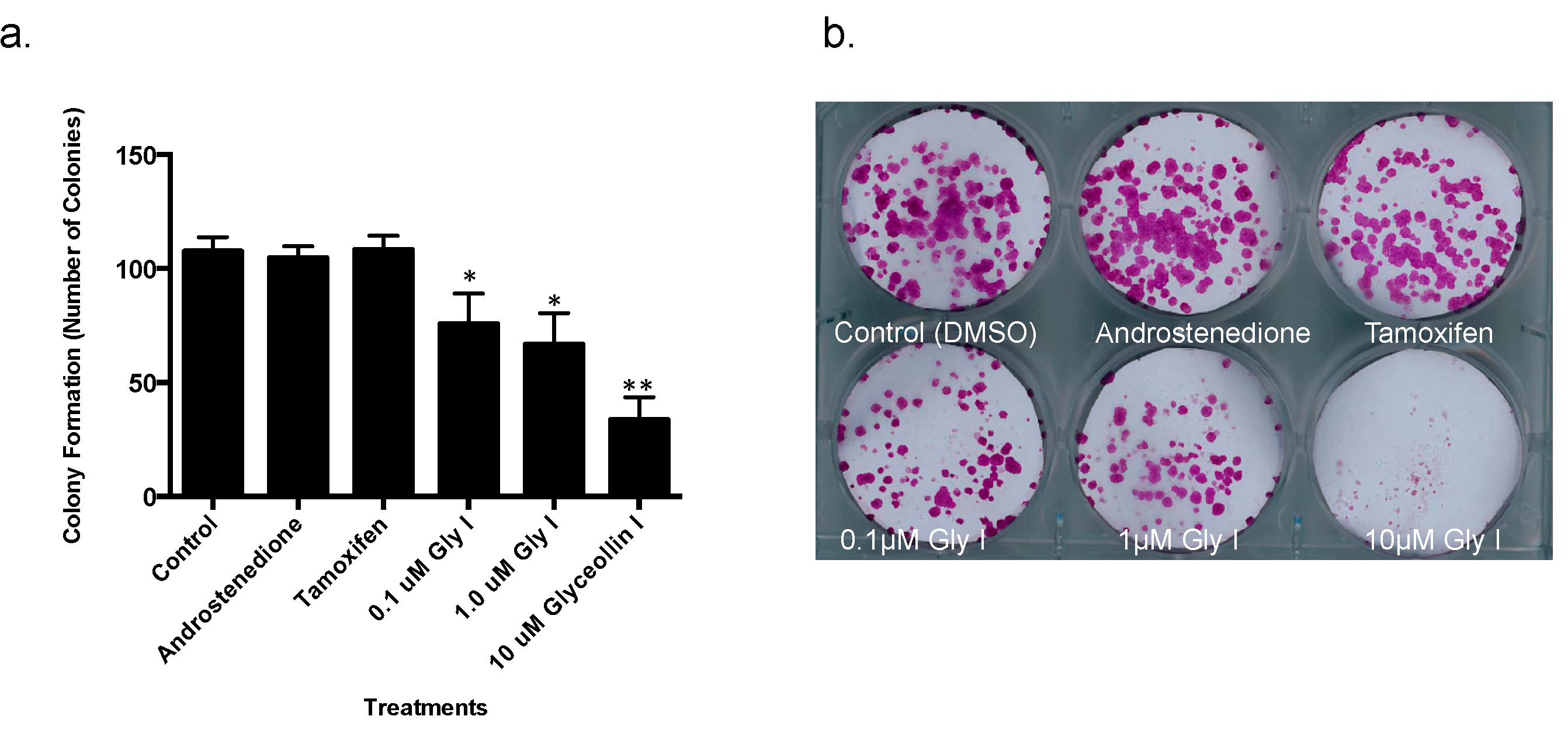
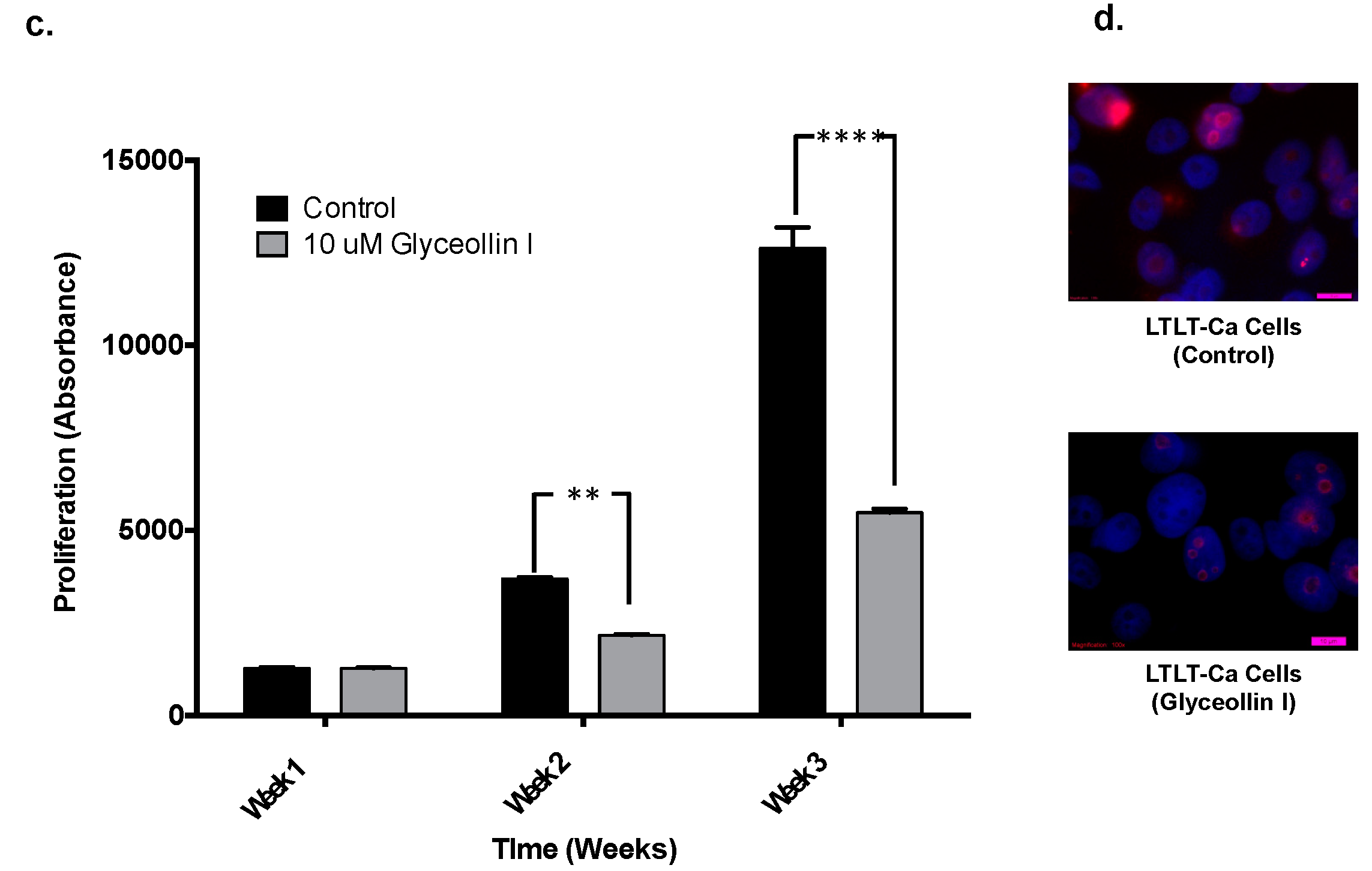
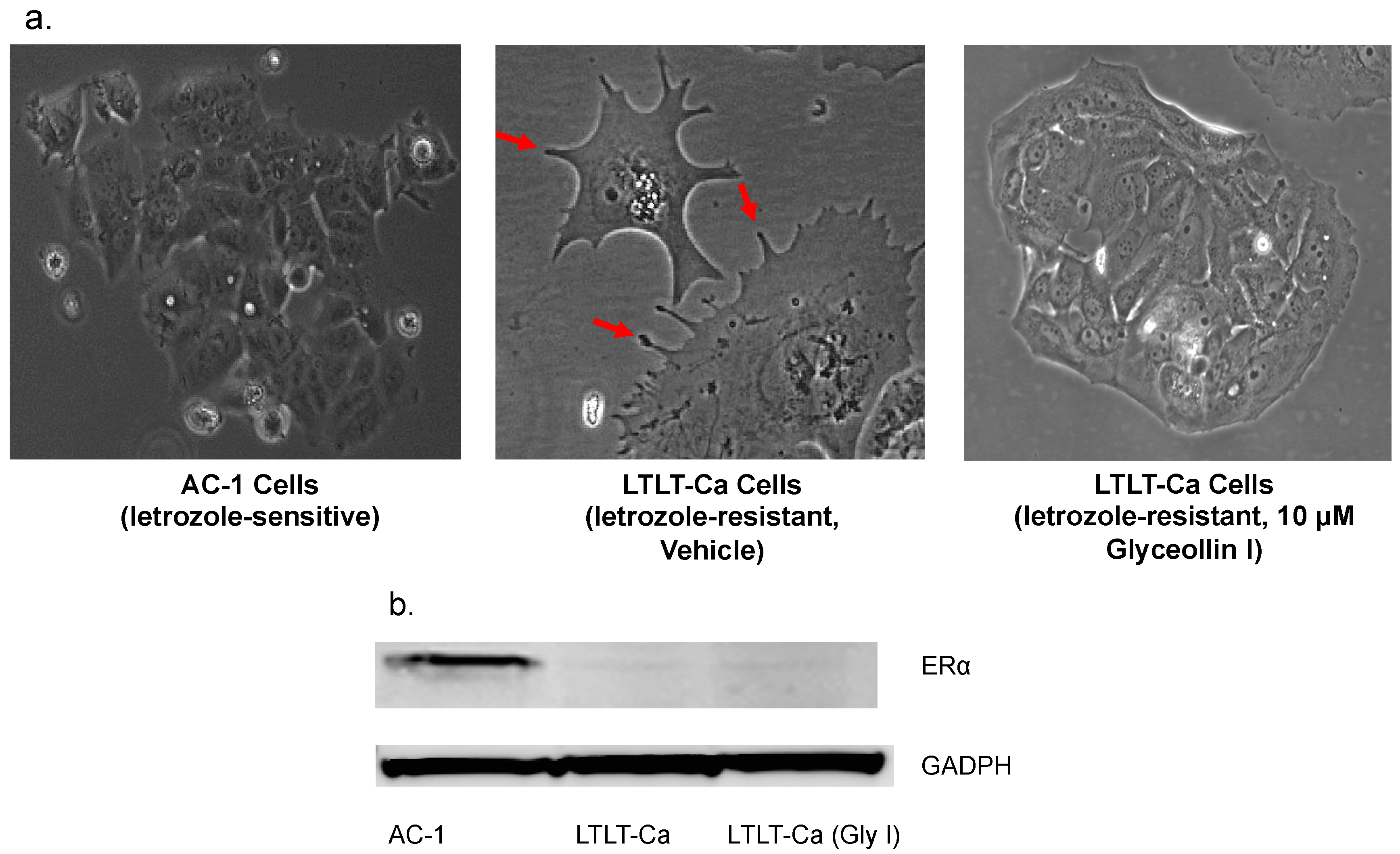
3.2. Glyceollin I Alters the Morphology of Letrozole-Resistant Breast Cancer Cells without Affecting ER Status
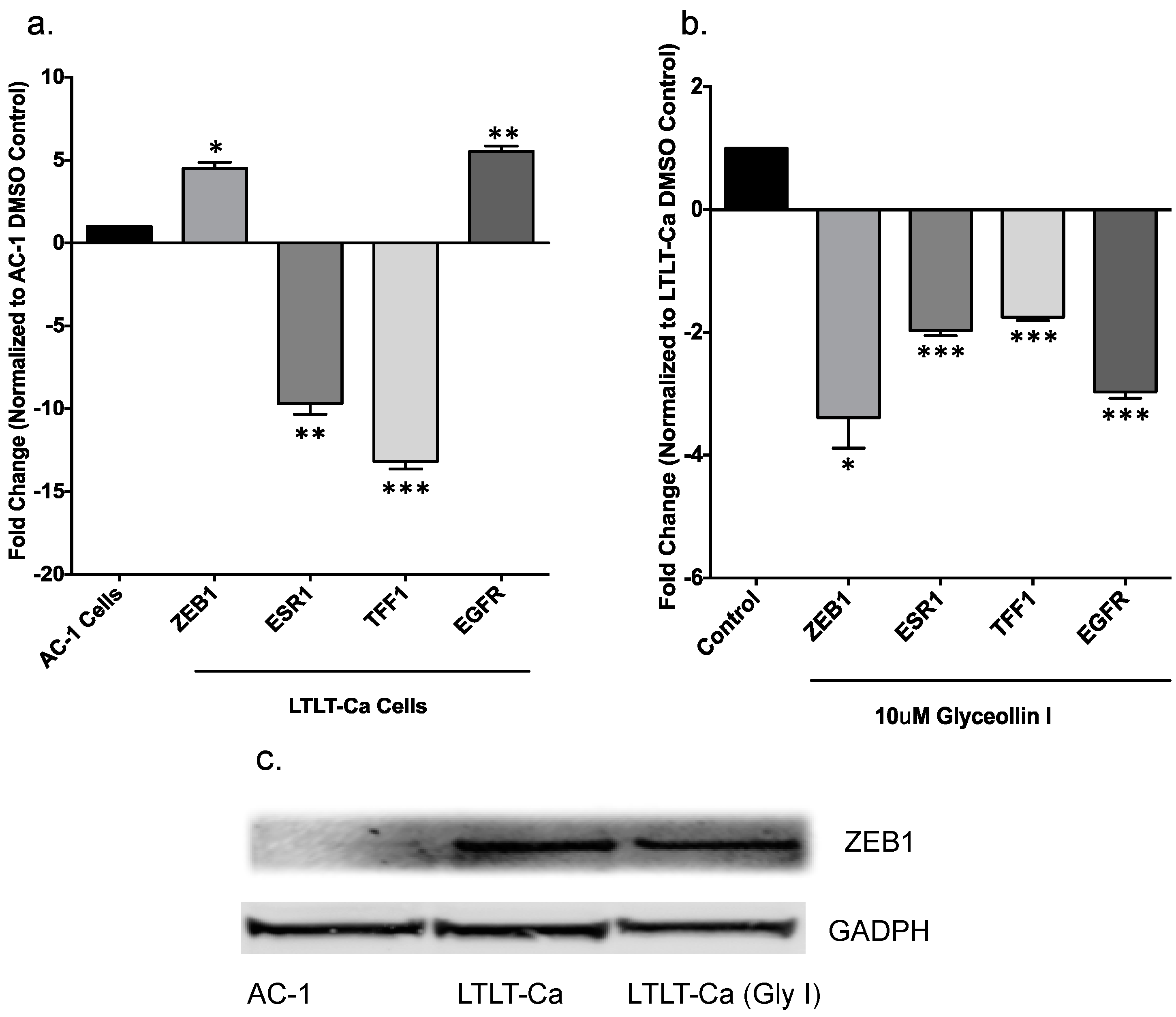
3.3. Glyceollin I Alters EMT Marker Expression in LTLT-Ca Cells in Vitro

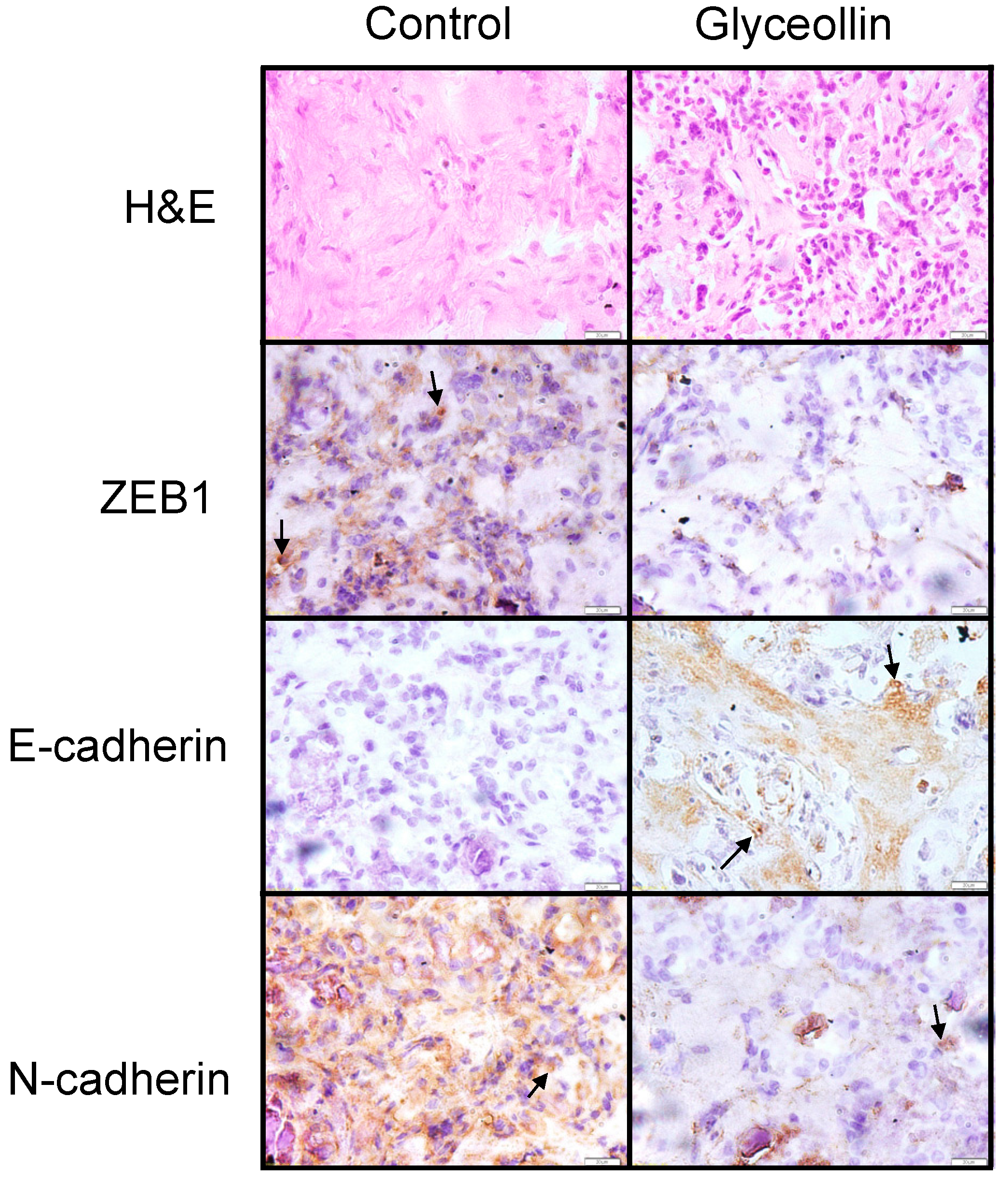
3.4. Glyceollin Inhibits the EMT-Like Phenotype in Vivo
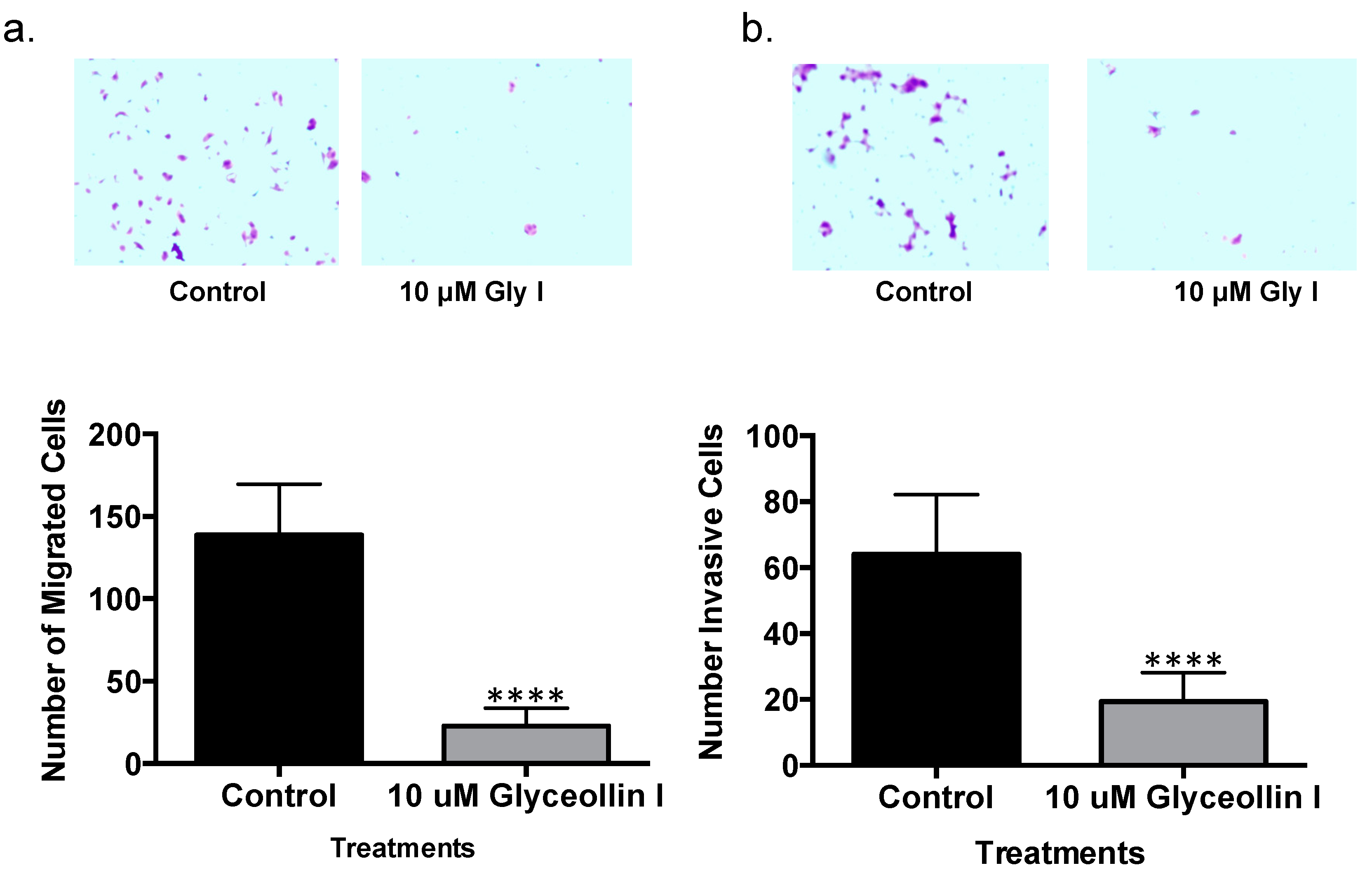
3.5. Glyceollin I Inhibits Letrozole-Resistant Cell Motility
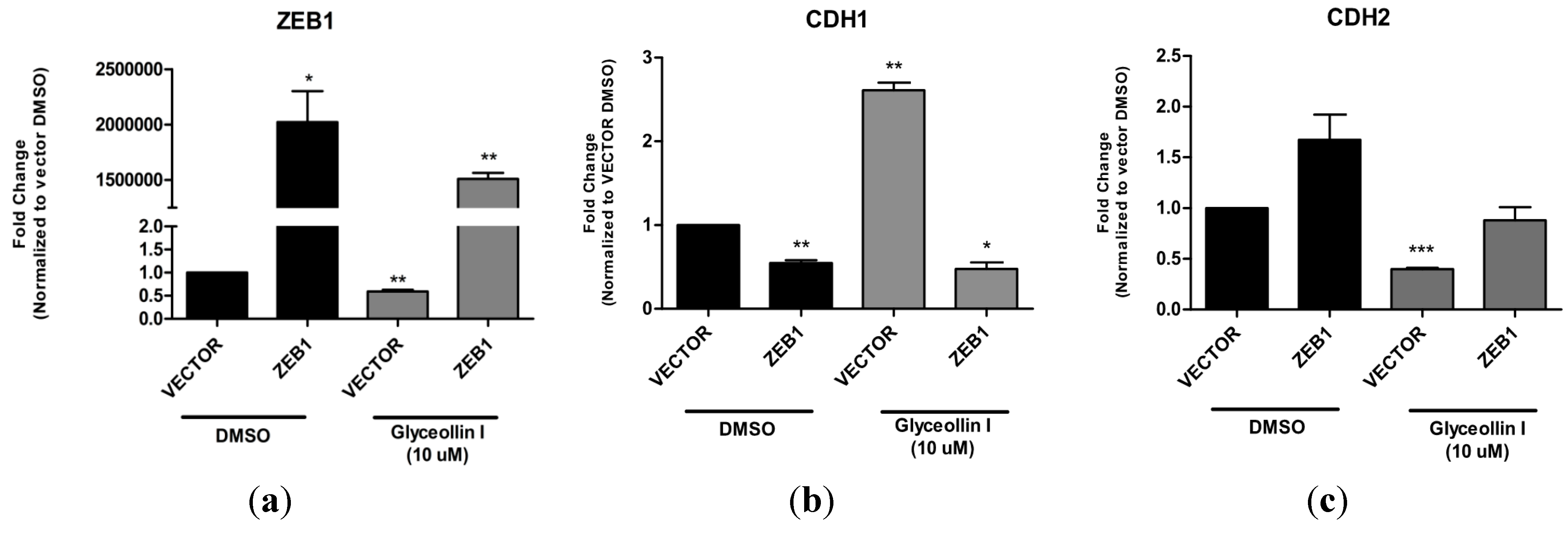
3.6. Forced Expression of ZEB1 Suppresses the Effects of Glyceollin I on EMT Markers
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, W.R.; Larionov, A.A. Understanding the mechanisms of aromatase inhibitor resistance. Breast Cancer Res.: BCR 2012, 14. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.; Martin, L.A.; Leary, A.; Head, J.; Dowsett, M. Clinical strategies for rationale combinations of aromatase inhibitors with novel therapies for breast cancer. J. Steroid Biochem. Mol. Biol. 2007, 106, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Kanaya, N.; Petrossian, K.; Ye, J.; Warden, C.; Liu, Z.; Nishimura, R.; Osako, T.; Okido, M.; Shimada, K.; et al. Inhibition of the proliferation of acquired aromatase inhibitor-resistant breast cancer cells by histone deacetylase inhibitor LBH589 (panobinostat). Breast Cancer Res. Treat. 2013, 137, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, G.; Brodie, A. Adaptive changes results in activation of alternate signaling pathways and resistance to aromatase inhibitor resistance. Mol. Cell. Endocrinol. 2011, 340, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, G.; Schayowitz, A.; Goloubeva, O.; Macedo, L.; Brodie, A. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. 2009, 69, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, G.; Goloubeva, O.; Gilani, R.; Macedo, L.; Brodie, A. Sensitivity to the aromatase inhibitor letrozole is prolonged after a “break” in treatment. Mol. Cancer Ther. 2010, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, G.J.; Macedo, L.F.; Goloubeva, O.; Schayowitz, A.; Brodie, A.M. Stopping treatment can reverse acquired resistance to letrozole. Cancer Res. 2008, 68, 4518–4524. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, G.J.; Goloubeva, O.G.; Kazi, A.A.; Shah, P.; Brodie, A.H. HDAC inhibitor entinostat restores responsiveness of letrozole-resistant MCF-7ca xenografts to aromatase inhibitors through modulation of Her-2. Mol. Cancer Ther. 2013, 12, 2804–2816. [Google Scholar] [CrossRef] [PubMed]
- Tilghman, S.L.; Townley, I.; Zhong, Q.; Carriere, P.P.; Zou, J.; Llopis, S.D.; Preyan, L.C.; Williams, C.C.; Skripnikova, E.; Bratton, M.R.; et al. Proteomic signatures of acquired letrozole resistance in breast cancer: Suppressed estrogen signaling and increased cell motility and invasiveness. Mol. Cell. Proteomics 2013, 12, 2440–2455. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Rubino, B.; Ballabio, G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology 2007, 39, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Berx, G.; Raspe, E.; Christofori, G.; Thiery, J.P.; Sleeman, J.P. Pre-emting metastasis? Recapitulation of morphogenetic processes in cancer. Clin. Exp. Metastasis 2007, 24, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Li, Y.; Wang, Z.; Sarkar, F.H. Cancer stem cells and epithelial-to-mesenchymal transition (emt)-phenotypic cells: Are they cousins or twins? Cancers 2011, 3, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Schmalhofer, O.; Brabletz, S.; Brabletz, T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009, 28, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Ganry, O. Phytoestrogen and breast cancer prevention. Eur. J. Cancer Prev. 2002, 11, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Limer, J.L.; Speirs, V. Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res. BCR 2004, 6, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilghman, S.L.; Boue, S.M.; Burow, M.E. Glyceollins, a novel class of antiestrogenic phytoalexins. Mol. Cell. Pharmacol. 2010, 2, 155–160. [Google Scholar]
- Kim, H.J.; Cha, B.Y.; Choi, B.; Lim, J.S.; Woo, J.T.; Kim, J.S. Glyceollins inhibit platelet-derived growth factor-mediated human arterial smooth muscle cell proliferation and migration. Br. J. Nutr. 2012, 107, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.; Jung, M.H.; Lee, Y.M. Glyceollins, a novel class of soy phytoalexins, inhibit angiogenesis by blocking the VEGF and BFGF signaling pathways. Mol. Nutr. Food Res. 2013, 57, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Sung, M.K.; Kim, J.S. Anti-inflammatory effects of glyceollins derived from soybean by elicitation with Aspergillus sojae. Inflamm. Res. 2011, 60, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Salvo, V.A.; Boue, S.M.; Fonseca, J.P.; Elliott, S.; Corbitt, C.; Collins-Burow, B.M.; Curiel, T.J.; Srivastav, S.K.; Shih, B.Y.; Carter-Wientjes, C.; et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin. Cancer Res. 2006, 12, 7159–7164. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.C.; Tilghman, S.L.; Boue, S.M.; Salvo, V.A.; Elliott, S.; Williams, K.Y.; Skripnikova, E.V.; Ashe, H.; Payton-Stewart, F.; Vanhoy-Rhodes, L.; et al. Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy. J. Pharmacol. Exp. Ther. 2010, 332, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.V.; Tilghman, S.L.; Boue, S.M.; Wang, S.; Khalili, H.; Muir, S.E.; Bratton, M.R.; Zhang, Q.; Wang, G.; Burow, M.E.; et al. Glyceollins as novel targeted therapeutic for the treatment of triple-negative breast cancer. Oncol. Lett. 2012, 3, 163–171. [Google Scholar] [PubMed]
- Jelovac, D.; Sabnis, G.; Long, B.J.; Macedo, L.; Goloubeva, O.G.; Brodie, A.M. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res. 2005, 65, 5380–5389. [Google Scholar] [CrossRef] [PubMed]
- Capes-Davis, A.; Reid, Y.A.; Kline, M.C.; Storts, D.R.; Strauss, E.; Dirks, W.G.; Drexler, H.G.; MacLeod, R.A.; Sykes, G.; Kohara, A.; et al. Match criteria for human cell line authentication: Where do we draw the line? Int. J. Cancer 2013, 132, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.P.; Yearby, L.A.; Stoute, D.; Burow, M.E.; Rhodes, L.V.; Gray, M.; Carriere, P.; Tilghman, S.L.; McLachlan, J.A.; Ochieng, J. In vitro and in vivo evaluation of novel anticancer agents in triple negative breast cancer models. J. Health Care Poor Underserved 2013, 24, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Lange, I.G.; Daxenberger, A.; Meyer, H.H. Tissue-specific expression pattern of estrogen receptors (ER): Quantification of ER alpha and ER beta mRNA with real-time RT-PCR. APMIS 2001, 109, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; de Goeij, A.F.; Rousch, M. Quality control in immunocytochemistry: Experiences with the oestrogen receptor assay. J. Clin. Pathol. 1992, 45, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Van Diest, P.J.; Weger, D.R.; Lindholm, J. Reproducibility of subjective immunoscoring of steroid receptors in breast cancer. Anal. Quant. Cytol. Histol. 1996, 18, 351–354. [Google Scholar] [PubMed]
- Tilghman, S.L.; Sabnis, G.; Brodie, A.M. Upregulation of AIB1, aromatase and eralpha provides long-term estrogen-deprived human breast cancer cells with a mechanistic growth advantage for survival. Horm. Mol. Biol. Clin. Investig. 2011, 3, 357–366. [Google Scholar] [PubMed]
- Schliekelman, M.J.; Gibbons, D.L.; Faca, V.M.; Creighton, C.J.; Rizvi, Z.H.; Zhang, Q.; Wong, C.H.; Wang, H.; Ungewiss, C.; Ahn, Y.H.; et al. Targets of the tumor suppressor MIR-200 in regulation of the epithelial-mesenchymal transition in cancer. Cancer Res. 2011, 71, 7670–7682. [Google Scholar] [CrossRef] [PubMed]
- Tilghman, S.L.; Rhodes, L.V.; Bratton, M.R.; Carriere, P.; Preyan, L.C.; Boue, S.M.; Vasaitis, T.S.; McLachlan, J.A.; Burow, M.E. Phytoalexins, mirnas and breast cancer: A review of phytochemical-mediated mirna regulation in breast cancer. J. Health Care Poor Underserved 2013, 24, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Hazan, R.B.; Kang, L.; Whooley, B.P.; Borgen, P.I. N-cadherin promotes adhesion between invasive breast cancer cells and the stroma. Cell Adhes. Commun. 1997, 4, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Hazan, R.B.; Phillips, G.R.; Qiao, R.F.; Norton, L.; Aaronson, S.A. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol. 2000, 148, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Buzdar, A.U. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: Analysis of survival and update of efficacy from the international letrozole breast cancer group. J. Clin. Oncol. 2004, 22, 3199–3200. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Gilani, R.A.; Kazi, A.A.; Shah, P.; Schech, A.J.; Chumsri, S.; Sabnis, G.; Jaiswal, A.K.; Brodie, A.H. The importance of her2 signaling in the tumor-initiating cell population in aromatase inhibitor-resistant breast cancer. Breast Cancer Res. Treat. 2012, 135, 681–692. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carriere, P.P.; Llopis, S.D.; Naiki, A.C.; Nguyen, G.; Phan, T.; Nguyen, M.M.; Preyan, L.C.; Yearby, L.; Pratt, J.; Burks, H.; et al. Glyceollin I Reverses Epithelial to Mesenchymal Transition in Letrozole Resistant Breast Cancer through ZEB1. Int. J. Environ. Res. Public Health 2016, 13, 10. https://doi.org/10.3390/ijerph13010010
Carriere PP, Llopis SD, Naiki AC, Nguyen G, Phan T, Nguyen MM, Preyan LC, Yearby L, Pratt J, Burks H, et al. Glyceollin I Reverses Epithelial to Mesenchymal Transition in Letrozole Resistant Breast Cancer through ZEB1. International Journal of Environmental Research and Public Health. 2016; 13(1):10. https://doi.org/10.3390/ijerph13010010
Chicago/Turabian StyleCarriere, Patrick P., Shawn D. Llopis, Anna C. Naiki, Gina Nguyen, Tina Phan, Mary M. Nguyen, Lynez C. Preyan, Letitia Yearby, Jamal Pratt, Hope Burks, and et al. 2016. "Glyceollin I Reverses Epithelial to Mesenchymal Transition in Letrozole Resistant Breast Cancer through ZEB1" International Journal of Environmental Research and Public Health 13, no. 1: 10. https://doi.org/10.3390/ijerph13010010





