An Investigation of Organic and Inorganic Mercury Exposure and Blood Pressure in a Small-Scale Gold Mining Community in Ghana
Abstract
:1. Introduction
| Study | n | Mean Hg | Population | SBP | DBP | Media |
|---|---|---|---|---|---|---|
| Park et al. (2013) [20] | 6607 | 1.03 a | US NHANES adults | − | + | Blood |
| Valera et al. (2009) [15] | 732 | 879 a | Canadian Nunavik Inuit adults | + | + | Blood |
| Goodrich et al. (2012) [16] | 262 | 0.45 | US Michigan dentists (adults) | + | + | Hair |
| Dorea et al. (2005) [25] | 296 | 3.4, 12.8 c | 4 Brazilian historic gold mining communities (adults) | + | + | Hair |
| Choi et al. (2009) [13] | 42 | 7.31 a | Farose whaling male adults | + | + | Hair |
| Fillion et al. (2006) [14] | 251 | 17.8 | 6 Brazilian communities (adults) | + | + | Hair |
| Park et al. (2013) [20] | 2201 | 0.51 a | US NHANES adults | − | + | Urine |
| Goodrich et al. (2012) [16] | 262 | 0.94 | US Michigan dentists (adults) | − | − | Urine |
| Siblerud (1990) [21] | 101 | 1.23, 3.7 d | US Utahan young adults | − | − | Urine |
| Kobal et al. (2004) [22] | 112 | 69.3 b | Slovenian male Hg miners (n = 54) & control | + | + | Urine |
2. Materials and Methods
2.1. Study Populations
2.2. Mercury Exposure Assessment
2.3. Blood Pressure and Pulse Assessment
2.4. BMI and Smoking
2.5. Statistical Analyses
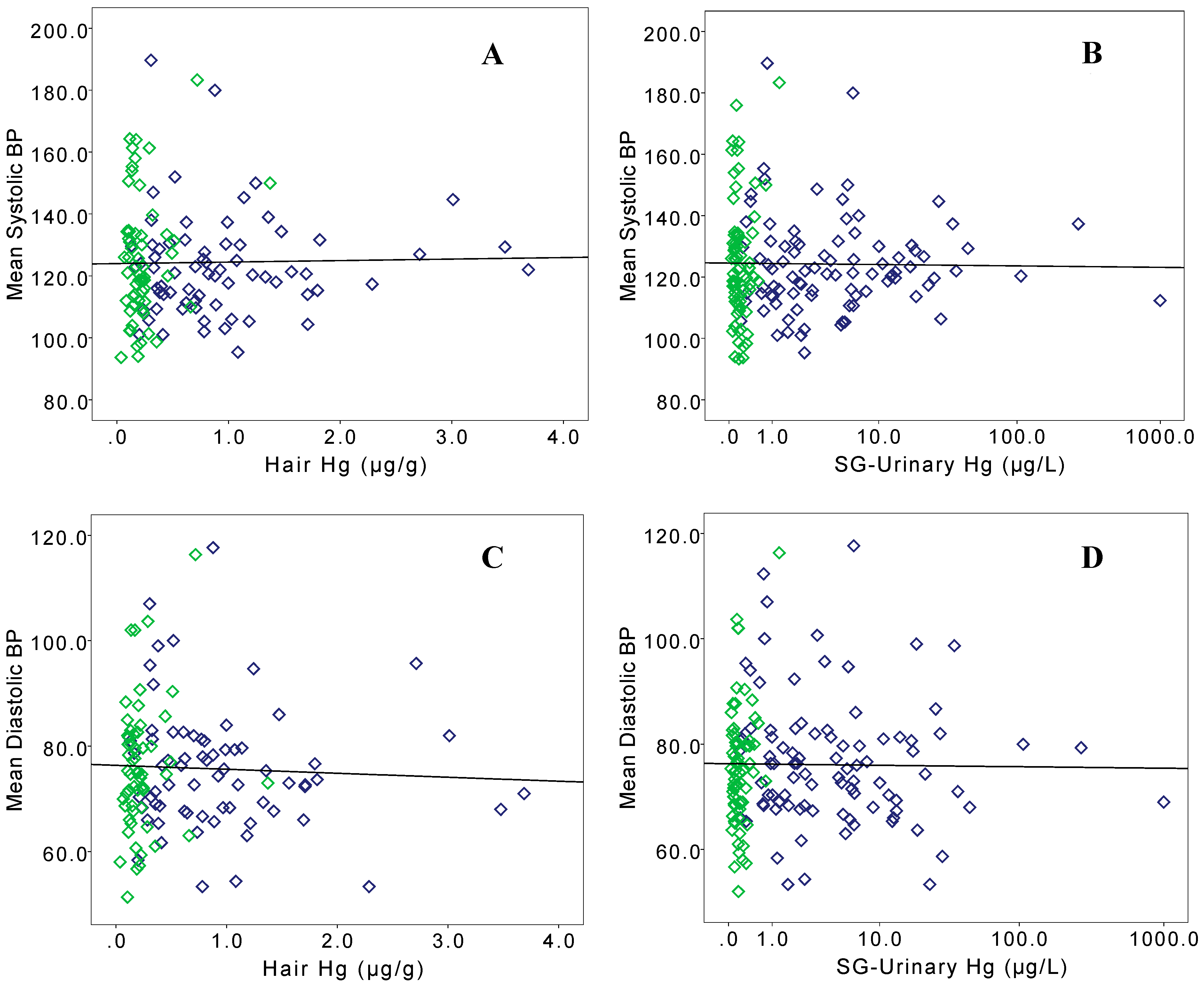
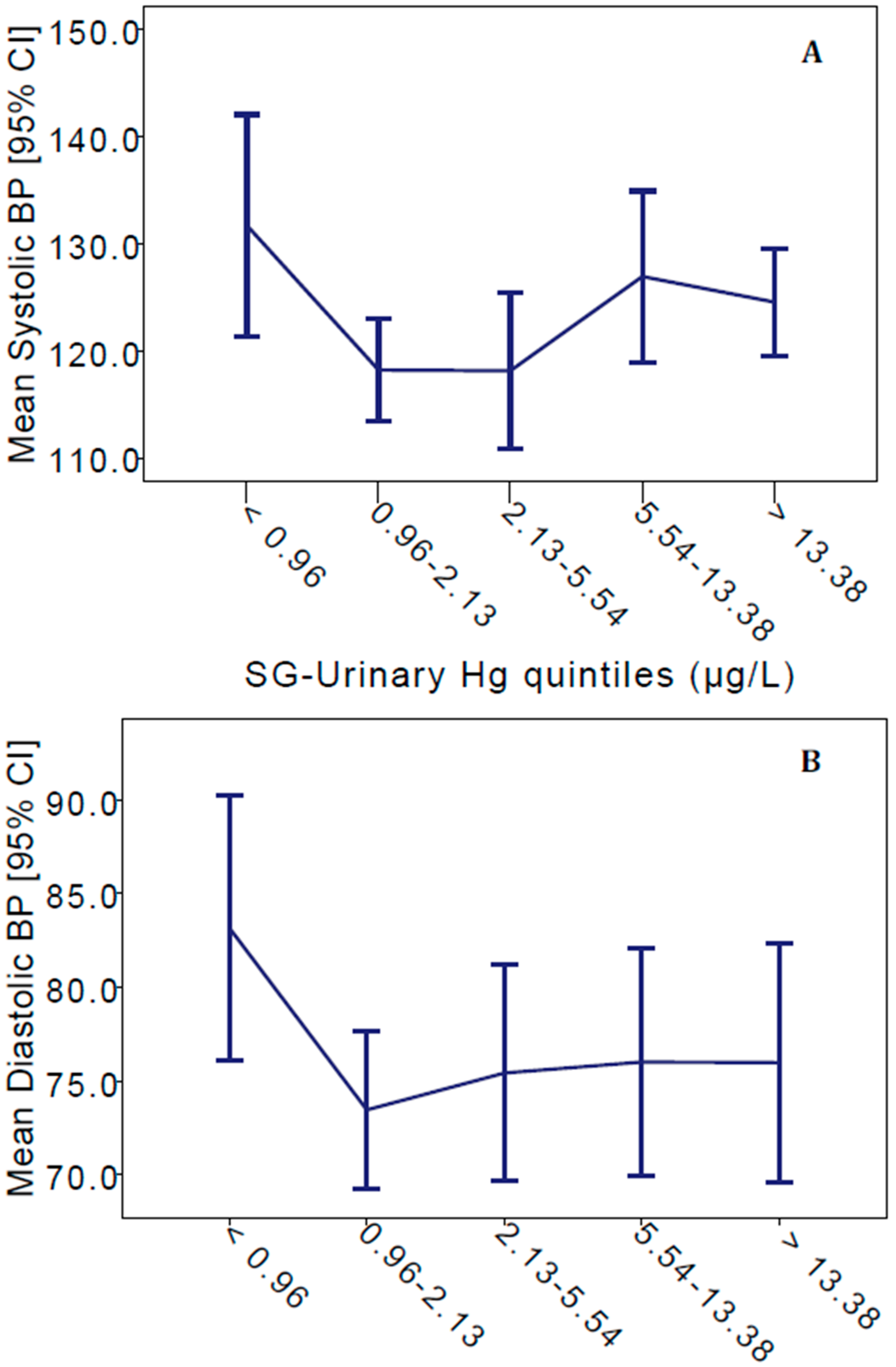
3. Results
| Kejetia | Gorogo | ||||||
|---|---|---|---|---|---|---|---|
| All | Miners a | Non-Miners a | |||||
| n participants | 96 | 70 | 26 | 75 | |||
| n households | 54 | 41 | 18 | 26 | |||
| Sex | % Male | 49 (51.0%) | 42 (60.0%) ** | 7 (26.9%) | 34 (45.3%) | ||
| Age | Mean (SD) | 31.4 (10.9) *** | 30.6 (9.7) | 33.8 (13.6) | 51.5 (18.8) | ||
| BMI b | |||||||
| <18.5 | 5 (5.2%) | 4 (5.7%) | 1 (3.8%) | 12 (16.0%) | |||
| 18.5 to 24.9 | 73 (76.0%) | 59 (84.3%) | 14 (53.8%) | 52 (69.3%) | |||
| 25 to 29.9 | 13 (13.5%) | 5 (7.1%) | 8 (30.8%) | 10 (13.3%) | |||
| 30 or higher | 5 (5.2%) | 2 (2.9%) | 3 (11.5%) | 1 (1.3%) | |||
| Mean (SD) | 22.7 (3.2) | 22.1 (2.7) ** | 24.5 (3.7) | 21.8 (3.1) | |||
| Smoking | |||||||
| Current smoker | 15 (15.6%) | 14 (20.0%) | 1 (3.8%) | 14 (18.7%) | |||
| Ex-smoker | 7 (7.3%) | 6 (8.6%) | 1 (3.8%) | 9 (12.0%) | |||
| n ever-smokers with pack-years c | 16 | 15 | 1 | 14 | |||
| Cigarette pack-years c | 15.8 (26.6) | 15.1 (27.4) | 25.5 | 3.9 (2.1) | |||
| Blood Pressure | Mean (SD) | ||||||
| Systolic BP (mmHg) | 123.5 (15.4) | 122.6 (12.4) | 125.8 (21.7) | 126.1 (20.0) | |||
| Diastolic BP (mmHg) | 76.7 (11.9) | 75.2 (10.3) * | 80.6 (14.9) | 75.4 (11.5) | |||
| Pulse Pressure d (mmHg) | 46.9 (9.3) * | 47.5 (8.2) | 45.2 (11.8) | 50.7 (13.3) | |||
| Mean Arterial Pressure e (mmHg) | 92.3 (12.4) | 91.0 (10.4) | 95.7 (16.5) | 92.3 (13.4) | |||
| Pulse | 77.1 (1.6) *** | 77.0 (1.5) | 77.2 (1.7) | 74.6 (3.2) | |||
| BP Classifications f | |||||||
| Normal | 38 (39.6%) | 27 (38.6%) | 11 (42.3%) | 30 (40.0%) | |||
| Prehypertension | 41 (42.7%) | 32 (45.7%) | 9 (34.6%) | 29 (38.7%) | |||
| Apparent hypertension | 17 (17.7%) | 11 (15.7%) | 6 (23.1%) | 16 (21.3%) | |||
| Hypertension Stage 1 | 12 (12.5%) | 9 (12.9%) | 3 (11.5%) | 8 (10.7%) | |||
| Hypertension Stage 2 | 5 (5.2%) | 2 (2.9%) | 3 (11.5%) | 8 (10.7%) | |||
| Kejetia | Gorogo | ||||
|---|---|---|---|---|---|
| All | Miners a | Non-Miners a | |||
| Urine | n | 91 | 67 | 24 | 70 |
| Urinary Specific Gravity (SG) | |||||
| Mean (SD) | 1.018 (0.007) *** | 1.017 (0.007) | 1.020 (0.006) | 1.014 (0.006) | |
| Urinary Hg (µg/L) | |||||
| Mean (SD) | 29.4 (148.6) *** | 37.6 (172.7) * | 6.61 (13.2) | 0.161 (0.131) | |
| Median | 2.71 | 4.24 | 1.41 | 0.114 | |
| IQR b | 1.03, 10.9 | 1.24, 11.0 | 0.742, 5.23 | 0.079, 0.217 | |
| Min–Max | 0.160–1372.3 | 0.160–1372.3 | 0.199–58.1 | 0.026–0.580 | |
| SG-adj. Urinary Hg c (µg/L) | |||||
| Mean (SD) | 21.7 (107.9) *** | 28.0 (125.3) *** | 4.22 (6.88) | 0.216 (0.194) | |
| Median | 3.10 | 5.17 | 1.18 | 0.154 | |
| IQR b | 1.13, 10.1 | 1.90, 12.5 | 0.733, 3.61 | 0.095, 0.261 | |
| Min–Max | 0.188–998.1 | 0.188–998.1 | 0.212–25.8 | 0.042–1.24 | |
| Hair Hg (µg/g) | n | 69 | 50 | 19 | 59 |
| Mean (SD) | 0.958 (0.742) *** | 1.11 (0.807) ** | 0.558 (0.272) | 0.231 (0.202) | |
| Median | 0.782 | 0.945 | 0.419 | 0.181 | |
| IQR b | 0.404, 1.20 | 0.571, 1.44 | 0.329, 0.718 | 0.119, 0.244 | |
| Min–Max | 0.132–3.69 | 0.132–3.69 | 0.237–1.10 | 0.037–1.37 | |
| Model | n | Adjusted r2 | Intercept | Hair Hg (µg/g) | Female | Current Smoker | Ex-Smoker | Age (Years) | |
|---|---|---|---|---|---|---|---|---|---|
| β | β (p-value) | ||||||||
| Systolic BP | Gorogo | 58 | 0.172 | 114.8 | 0.748 (0.95) | −13.4 (0.07) | −15.7 (0.06) | −13.8 (0.16) | 0.433 (0.001) |
| Kejetia | 67 | 0.350 | 112.2 | 1.45 (0.41) | −13.7 (<0.001) | −14.2 (0.004) | −7.91 (0.17) | 0.600 (<0.001) | |
| Kejetia: Current miners | 50 | 0.283 | 109.6 | 1.40 (0.49) | −11.3 (0.005) | −13.3 (0.017) | −6.73 (0.30) | 0.648 (<0.001) | |
| Diastolic BP | Gorogo | 58 | −0.016 | 71.3 | −1.71 (0.83) | −3.63 (0.44) | −3.60 (0.49) | −4.47 (0.48) | 0.150 (0.07) |
| Kejetia | 68 | 0.338 | 60.0 | −0.696 (0.65) | −4.11 (0.13) | −9.27 (0.027) | 1.59 (0.75) | 0.636 (<0.001) | |
| Kejetia: Current miners | 50 | 0.285 | 60.0 | −0.508 (0.75) | −3.80 (0.21) | −8.23 (0.06) | 2.44 (0.62) | 0.601 (<0.001) | |
| Pulse pressure a | Gorogo | 58 | 0.184 | 43.4 | 2.46 (0.76) | −9.74 (0.049) | −12.1 (0.029) | −9.30 (0.16) | 0.283 (0.002) |
| Kejetia | 67 | 0.197 | 50.4 | 2.09 (0.15) | −9.45 (<0.001) | −5.51 (0.16) | −9.64 (0.040) | 0.030 (0.79) | |
| Kejetia: Current miners | 50 | 0.101 | 49.6 | 1.91 (0.23) | −7.47 (0.015) | −5.11 (0.23) | −9.17 (0.07) | 0.047 (0.72) | |
| Mean arterial pressure b | Gorogo | 58 | 0.076 | 85.8 | −0.890 (0.92) | −6.85 (0.19) | −7.61 (0.19) | −7.55 (0.28) | 0.245 (0.009) |
| Kejetia | 67 | 0.316 | 78.6 | 0.057 (0.97) | −7.44 (0.005) | −10.5 (0.010) | −1.48 (0.75) | 0.580 (<0.001) | |
| Kejetia: Current miners | 50 | 0.315 | 76.5 | 0.126 (0.94) | −6.30 (0.040) | −9.93 (0.02) | −0.615 (0.90) | 0.617 (<0.001) | |
| Pulse | Gorogo | 58 | 0.159 | 74.7 | 3.22 (0.13) | 0.267 (0.83) | 1.64 (0.24) | −3.74 (0.030) | −0.009 (0.67) |
| Kejetia | 67 | 0.066 | 79.4 | −0.328 (0.26) | −0.874 (0.09) | −0.102 (0.90) | −1.72 (0.07) | −0.048 (0.038) | |
| Kejetia: Current miners | 50 | −0.015 | 78.6 | −0.333 (0.29) | −0.571 (0.34) | −0.114 (0.89) | −1.57 (0.12) | −0.026 (0.33) | |
| Model | n | Adjusted r2 | Intercept | SG-Urinary Hg (µg/L) | Female | Current Smoker | Ex-Smoker | Age (Years) | |
|---|---|---|---|---|---|---|---|---|---|
| β | β (p-value) | ||||||||
| Systolic BP | Kejetia | 86 | 0.216 | 114.6 | 0.010 (0.94) | −10.6 (0.001) | −5.28 (0.19) | −10.0 (0.046) | 0.466 (<0.001) |
| Kejetia: Current miners | 64 | 0.244 | 108.3 | 0.098 (0.52) | −9.35 (0.009) | −5.73 (0.18) | −8.63 (0.11) | 0.622 (<0.001) | |
| Diastolic BP | Kejetia | 86 | 0.279 | 62.1 | −0.087 (0.46) | −4.31 (0.09) | −1.07 (0.75) | −3.03 (0.46) | 0.533 (<0.001) |
| Kejetia: Current miners | 64 | 0.303 | 59.1 | −0.106 (0.40) | −3.77 (0.19) | −0.263 (0.94) | −1.44 (0.74) | 0.595 (<0.001) | |
| Pulse pressure a | Kejetia | 85 | 0.109 | 50.6 | 0.116 (0.25) | −6.85 (0.002) | −4.77 (0.10) | −7.30 (0.041) | −0.006 (0.95) |
| Kejetia: Current miners | 64 | 0.112 | 49.2 | 0.205 (0.07) | −5.57 (0.028) | −5.47 (0.07) | −7.19 (0.06) | 0.026 (0.81) | |
| Mean arterial pressure b | Kejetia | 85 | 0.266 | 80.6 | −0.046 (0.68) | −6.82 (0.006) | −2.13 (0.50) | −5.11 (0.20) | 0.473 (<0.001) |
| Kejetia: Current miners | 64 | 0.302 | 75.5 | −0.038 (0.76) | −5.64 (0.05) | −2.08 (0.55) | −3.83 (0.38) | 0.604 (<0.001) | |
| Pulse | Kejetia | 85 | 0.055 | 78.6 | −0.029 (0.15) | −0.766 (0.08) | 0.003 (0.995) | −1.36 (0.05) | −0.028 (0.10) |
| Kejetia: Current miners | 64 | 0.005 | 77.9 | −0.016 (0.47) | −0.254 (0.61) | 0.176 (0.77) | −1.39 (0.07) | −0.019 (0.40) | |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R.; Choi, A.L.; Oken, E.; Horvat, M.; Schoeny, R.; Kamai, E.; Cowell, W.; Grandjean, P.; Korrick, S. Evidence on the human health effects of low-level methylmercury exposure. Environ. Health Perspect. 2012, 120, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin. Hypertens. 2011, 13, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Wiggers, G.A.; Peçanha, F.M.; Briones, A.M.; Pérez-Girón, J.V.; Miguel, M.; Vassallo, D.V.; Cachofeiro, V.; Alonso, M.J.; Salaices, M. Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1033–H1043. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Seppänen, K.; Nyyssönen, K.; Korpela, H.; Kauhanen, J.; Kantola, M.; Tuomilehto, J.; Esterbauer, H.; Tatzber, F.; Salonen, R. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 1995, 91, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M.; Vanhoutte, P.M. Endothelial dysfunction : A multifaceted disorder. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H985–H1002. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Waack, B.J.; Weno, B.L.; Heistad, D.D. Increases in pulse pressure impair acetylcholine-induced vascular relaxation. Am. Physiol. Soc. 1995, 268, 359–363. [Google Scholar]
- Ceravolo, R.; Maio, R.; Pujia, A.; Sciacqua, A.; Ventura, G.; Costa, M.C.; Sesti, G.; Perticone, F. Pulse pressure and endothelial dysfunction in never-treated hypertensive patients. J. Am. Coll. Cardiol. 2003, 41, 1753–1758. [Google Scholar] [CrossRef]
- Rhee, H.M.; Choi, B.H. Hemodynamic and electrophysiological effects of mercury in intact anesthetized rabbits and in isolated perfused hearts. Exp. Mol. Pathol. 1989, 50, 281–290. [Google Scholar] [CrossRef]
- Norn, S.; Permin, H.; Kruse, E.; Kruse, P.R. Mercury—A major agent in the history of medicine and alchemy. Med. Hist. 2008, 36, 21–40. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Mercury Study Report to Congress: Health Effects of Mercury and Mercury Compounds, Volume 5; EPA: Washington, DC, USA, 1997.
- Choi, A.L.; Weihe, P.; Budtz-jørgensen, E.; Jørgensen, P.J.; Jukka, T.; Tuomainen, T.; Murata, K.; Nielsen, H.P.; Skaalum, M.; Askham, J.; et al. Methylmercury Exposure in Faroese Whaling Men and Adverse Cardiovascular Effects. Environ. Health Perspect. 2009, 117, 367–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillion, M.; Mergler, D.; Passos, C.J.S.; Larribe, F.; Lemire, M.; Guimaraes, J.R.D. A preliminary study of mercury exposure and blood pressure in the Brazilian Amazon. Environ. Health A Glob. Access Sci. Source 2006, 5, 1–9. [Google Scholar]
- Valera, B.; Dewailly, É.; Poirier, P. Environmental mercury exposure and blood pressure among Nunavik inuit adults. Hypertension 2009, 54, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.M.; Wang, Y.; Gillespie, B.; Werner, R.; Franzblau, A.; Basu, N. Methylmercury and elemental mercury differentially associate with blood pressure among dental professionals. Int. J. Hyg. Environ. Health 2012, 216, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, R.E.; Bobbitt, R.G.; Welch, L.W.; Wood, A.J.J.; Bonfiglio, J.F.; Sarzen, C.; Heath, A.J.; Branch, R.A. Elemental Mercury Vapour Toxicity, Treatment, and Prognosis After Acute, Intensive Exposure in Chloralkali Plant Workers. Part I: History, Neuropsychological Findings and Chelator effects. Hum. Exp. Toxicol. 1992, 11, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Massaroni, L.; Rossoni, L.V.; Amaral, S.M.; Stefanon, I.; Oliveira, E.M.; Vassallo, D.V. Haemodynamic and electrophysiological acute toxic effects of mercury in anaesthetized rats and in langendorff perfused rat hearts. Pharmacol. Res. 1995, 32, 27–36. [Google Scholar] [CrossRef]
- Rossoni, L.V.; Amaral, S.M.; Vassallo, P.F.; França, A.; Oliveira, E.M.; Varner, K.J.; Mill, J.G.; Vassallo, D.V. Effects of mercury on the arterial blood pressure of anesthetized rats. Braz. J. Med. Biol. Res. 1999, 32, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Lee, S.; Basu, N.; Franzblau, A. Associations of blood and urinary mercury with hypertension in U.S. Adults: The NHANES 2003–2006. Environ. Res. 2013, 123, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Siblerud, R.L. The relationship between mercury from dental amalgam and the cardiovascular system. Sci. Total Environ. 1990, 99, 23–35. [Google Scholar] [CrossRef]
- Kobal, A.B.; Horvat, M.; Prezelj, M.; Briški, A.S.; Krsnik, M.; Dizdarevič, T.; Mazej, D.; Falnoga, I.; Stibilj, V.; Arnerič, N.; et al. The impact of long-term past exposure to elemental mercury on antioxidative capacity and lipid peroxidation in mercury miners. J. Trace Elem. Med. Biol. 2004, 17, 261–274. [Google Scholar] [CrossRef]
- Center for Disease Control (CDC). Fourth National Report on Human Exposure to Environmental Chemicals; CDC: Atlanta, GA, USA, 2009.
- Nicolae, A.; Ames, H.; Quiñonez, C. Dental amalgam and urinary mercury concentrations: A descriptive study. BMC Oral Health 2013, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G.; De Souza, J.R.; Rodrigues, P.; Ferrari, Í.; Barbosa, A.C. Hair mercury (signature of fish consumption) and cardiovascular risk in Munduruku and Kayabi Indians of Amazonia. Environ. Res. 2005, 97, 209–219. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme (UNEP). Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport; UNEP: Geneva, Switzerland, 2013. [Google Scholar]
- Basu, N.; Clarke, E.; Green, A.; Long, R.; Calys-Tagoe, B.; Chan, L.H.M.; Dzodzomenyo, M.; Fobil, J.N.; Neitzel, R.L.; Obiri, S.; Odei, E.; et al. Integrated Assessment of Artisanal and Small-Scale Gold Mining in Ghana—Part 1: Human Health Review. Int. J. Environ. Res. Public Health 2015, 12, 5143–5176. [Google Scholar] [CrossRef] [PubMed]
- Wranová, K.; Čejchanová, M.; Spěváčková, V.; Korunová, V.; Vobecký, M.; Spěváček, V. Mercury and methylmercury in hair of selected groups of Czech population. Cent. Eur. J. Public Health 2008, 17, 36–40. [Google Scholar]
- Barbosa, A.C.; Boischio, A.A.; East, G.A.; Ferrari, I.; Gonçalves, A.; Silva, P.R.M.; da Cruz, T.M.E. Mercury contamination in the Brazilian Amazon. Environmental and occupational aspects. Water Air Soil Pollut. 1995, 80, 109–121. [Google Scholar] [CrossRef]
- Bosu, W.K. Epidemic of hypertension in Ghana: A systematic review. BMC Public Health 2010. [Google Scholar] [CrossRef] [PubMed]
- Pobee, J.O.; Larbi, E.B.; Belcher, D.W.; Wurapa, F.K.; Dodu, S.R. Blood pressure distribution in a rural Ghanaian population. Trans. R. Soc. Trop. Med. Hyg. 1977, 71, 66–72. [Google Scholar] [CrossRef]
- Paruchuri, Y.; Siuniak, A.; Johnson, N.; Levin, E.; Mitchell, K.; Goodrich, J.M.; Renne, E.P.; Basu, N. Occupational and environmental mercury exposure among small-scale gold miners in the Talensi-Nabdam District of Ghana’s Upper East region. Sci. Total Environ. 2010, 408, 6079–6085. [Google Scholar] [CrossRef] [PubMed]
- Ghana Statistical Service (GSS); Ghana Health Service (GHS); ICF Macro. Ghana Demographic and Health Survey 2008; GSS: Accra, Ghana; GHS: Accra, Ghana; Calverton, MD, USA, 2009. [Google Scholar]
- Ferris, B.G. Epidemiology Standardization Project. Am. Thorac. Soc. 1978, 118, 1–120. [Google Scholar]
- Hoshaw-Woodard, S. Description and Comparison of the Methods of Cluster Sampling and Lot Quality Assurance Sampling to Assess Immunization Coverage; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry; EPA: Washington, DC, USA, 2007.
- Rajaee, M.; Long, R.N.; Renne, E.P.; Basu, N. Mercury concentrations and spatial distribution in a Ghanaian small-scale gold mining community. In preparation. 2015. [Google Scholar]
- Lee, E.; Park, H.K.; Kim, H.J. Adjustment of urinary mercury in health risk assessment of mercury. J. Korea Med. Sci. 1996, 11, 319–325. [Google Scholar] [CrossRef]
- Voinescu, G.C.; Shoemaker, M.; Moore, H.; Khanna, R.; Nolph, K.D. The relationship between urine osmolality and specific gravity. Am. J. Med. Sci. 2002, 323, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Understanding Blood Pressure Readings. Available online: http://www.heart.org/HEARTORG/Conditions/HighBloodPressure/AboutHighBloodPressure/Understanding-Blood-Pressure-Readings_UCM_301764_Article.jsp (accessed on 10 January 2015).
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the American Heart Association cou. Hypertension 2005, 45, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Razminia, M.; Trivedi, A.; Molnar, J.; Elbzour, M.; Guerrero, M.; Salem, Y.; Ahmed, A.; Khosla, S.; Lubell, D.L. Validation of a new formula for mean arterial pressure calculation: The new formula Is superior to the standard formula. Catheter. Cardiovasc. Interv. 2004, 63, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, G.; Laird, N.; Ware, J. Applied Longitudinal Analysis, 2nd ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Rajaee, M.; Obiri, S.; Green, A.; Long, R.; Cobbina, S.; Nartey, V.; Buck, D.; Antwi, E.; Basu, N. Integrated Assessment of Artisanal and Small-Scale Gold Mining in Ghana—Part 2: Natural Sciences Review. Int. J. Environ. Resesearch Public Health 2015, 12, 8971–9011. [Google Scholar] [CrossRef] [PubMed]
- Xun, P.; Hou, N.; Daviglus, M.; Liu, K.; Morris, J.S.; Shikany, J.M.; Sidney, S.; Jacobs, D.R.; He, K. Fish oil, selenium and mercury in relation to incidence of hypertension: A 20-year follow-up study. J. Intern. Med. 2011, 270, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.S.; Blum, J.D.; Basu, N.; Rajaee, M.; Evers, D.C.; Buck, D.G.; Petrlik, J.; Digangi, J. Assessment of mercury exposure among small-scale gold miners using mercury stable isotopes. Environ. Res. 2015, 137, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Gibb, H.; O’Leary, K.G. Mercury Exposure and Health Impacts among Individuals in the Artisanal and Small-Scale Gold Mining Community: A Comprehensive Review. Environ. Health Perspect. 2014, 122, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, I.; Kotchen, J.M.; Kotchen, T.A. Hypertension: trends in prevalence, incidence, and control. Annu. Rev. Public Health 2006, 27, 465–490. [Google Scholar] [CrossRef] [PubMed]
- Valera, B.; Dewailly, E.; Poirier, P. Cardiac autonomic activity and blood pressure among Nunavik Inuit adults exposed to environmental mercury: A cross-sectional study. Environ. Health 2008. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.B.; Jørgensen, M.E.; Pedersen, M.B.; Siggaard, C.; Sørensen, T.B.; Mulvad, G.; Hansen, J.C.; Asmund, G.; Skjoldborg, H. Relationship between mercury in blood and 24-h ambulatory blood pressure in greenlanders and Danes. Am. J. Hypertens. 2005, 18, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Dart, A.M.; Kingwell, B.A. Pulse pressure—A review of mechanisms and clinical relevance. J. Am. Coll. Cardiol. 2001, 37, 975–984. [Google Scholar] [CrossRef]
- Sesso, H.D.; Stampfer, M.J.; Rosner, B.; Hennekens, C.H.; Gaziano, J.M.; Manson, J.E.; Glynn, R.J. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension 2000, 36, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Valera, B.; Dewailly, E.; Poirier, P.; Counil, E.; Suhas, E. Influence of mercury exposure on blood pressure, resting heart rate and heart rate variability in French Polynesians: A cross-sectional study. Environ. Health 2011. [Google Scholar] [CrossRef] [PubMed]
- Valera, B.; Muckle, G.; Poirier, P.; Jacobson, S.W.; Jacobson, J.L.; Dewailly, E. Cardiac autonomic activity and blood pressure among Inuit children exposed to mercury. Neurotoxicology 2012, 33, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Bawa, I. A viewpoint on small-scale gold mining in Ghana: A regulatory perspective on current practices, mercury use and the UNIDO and EU projects. Int. J. Environ. Pollut. 2010, 41, 195–201. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajaee, M.; Sánchez, B.N.; Renne, E.P.; Basu, N. An Investigation of Organic and Inorganic Mercury Exposure and Blood Pressure in a Small-Scale Gold Mining Community in Ghana. Int. J. Environ. Res. Public Health 2015, 12, 10020-10038. https://doi.org/10.3390/ijerph120810020
Rajaee M, Sánchez BN, Renne EP, Basu N. An Investigation of Organic and Inorganic Mercury Exposure and Blood Pressure in a Small-Scale Gold Mining Community in Ghana. International Journal of Environmental Research and Public Health. 2015; 12(8):10020-10038. https://doi.org/10.3390/ijerph120810020
Chicago/Turabian StyleRajaee, Mozhgon, Brisa N. Sánchez, Elisha P. Renne, and Niladri Basu. 2015. "An Investigation of Organic and Inorganic Mercury Exposure and Blood Pressure in a Small-Scale Gold Mining Community in Ghana" International Journal of Environmental Research and Public Health 12, no. 8: 10020-10038. https://doi.org/10.3390/ijerph120810020





