Acute Effects of Exposure to a Traditional Rural Environment on Urban Dwellers: A Crossover Field Study in Terraced Farmland
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects and Study Sites

2.2. Experimental Design
| Rural | Urban | Differences | |||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Physiological parameters | |||||
| Pulse rate(bpm) | 59.1 | 3.0 | 61.5 | 3.6 | ns |
| SBP(mmHg) a | 116.0 | 2.1 | 122.2 | 3.5 | ns |
| DBP(mmHg) b | 61.7 | 1.9 | 64.1 | 2.0 | ns |
| ln(HF) | 6.5 | 0.2 | 6.1 | 0.4 | ns |
| ln(LF/HF) | −2.3 | 0.7 | −3.1 | 0.8 | ns |
| Psychological parameters | |||||
| SD | |||||
| Comfortable feeling | 2.5 | 0.5 | 1.5 | 0.5 | ns |
| Soothed feeling | 1.9 | 0.7 | 2.3 | 0.7 | ns |
| Natural feeling | −1.2 | 1.0 | −1.0 | 0.8 | ns |
| Refreshed feeling | 47.5 | 5.0 | 52.7 | 4.1 | ns |
| POMS | |||||
| Tension-anxiety | 46.7 | 3.2 | 44.7 | 4.4 | ns |
| Depression | 46.8 | 3.3 | 47.2 | 3.0 | ns |
| Anger-hostility | 43.8 | 2.8 | 41.5 | 1.9 | ns |
| Fatigue | 47.4 | 3.9 | 46.4 | 4.2 | ns |
| Confusion | 47.5 | 2.7 | 49.4 | 3.8 | ns |
| Vigor | 43.1 | 2.6 | 40.9 | 2.6 | ns |
2.3. Measurement
2.3.1. Physiological Parameters
2.3.2. Questionnaires
2.3.3. Air Quality Analysis
2.4. Data Analysis
3. Results
3.1. Physiological Parameters



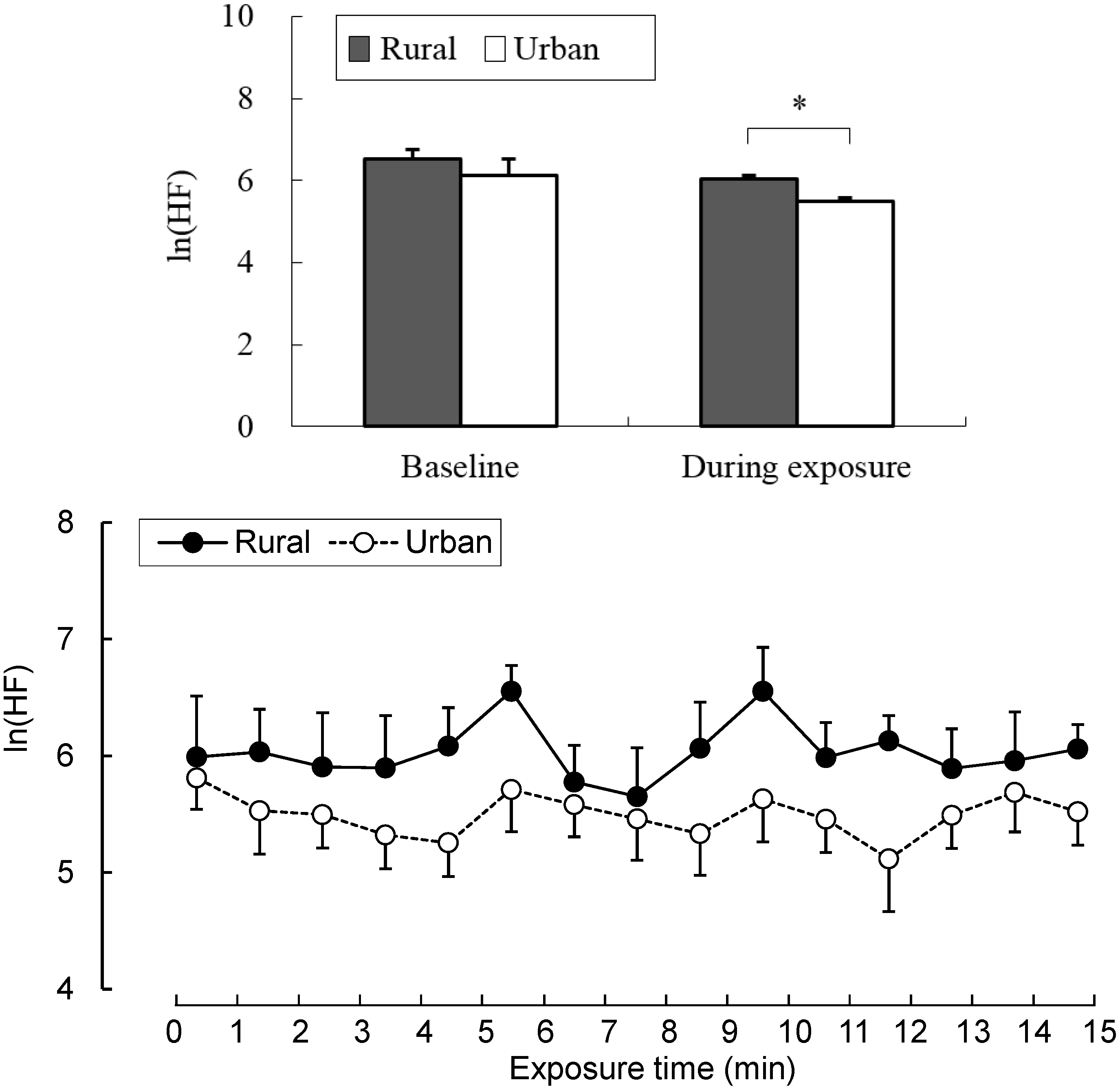

3.2. Psychological Parameters
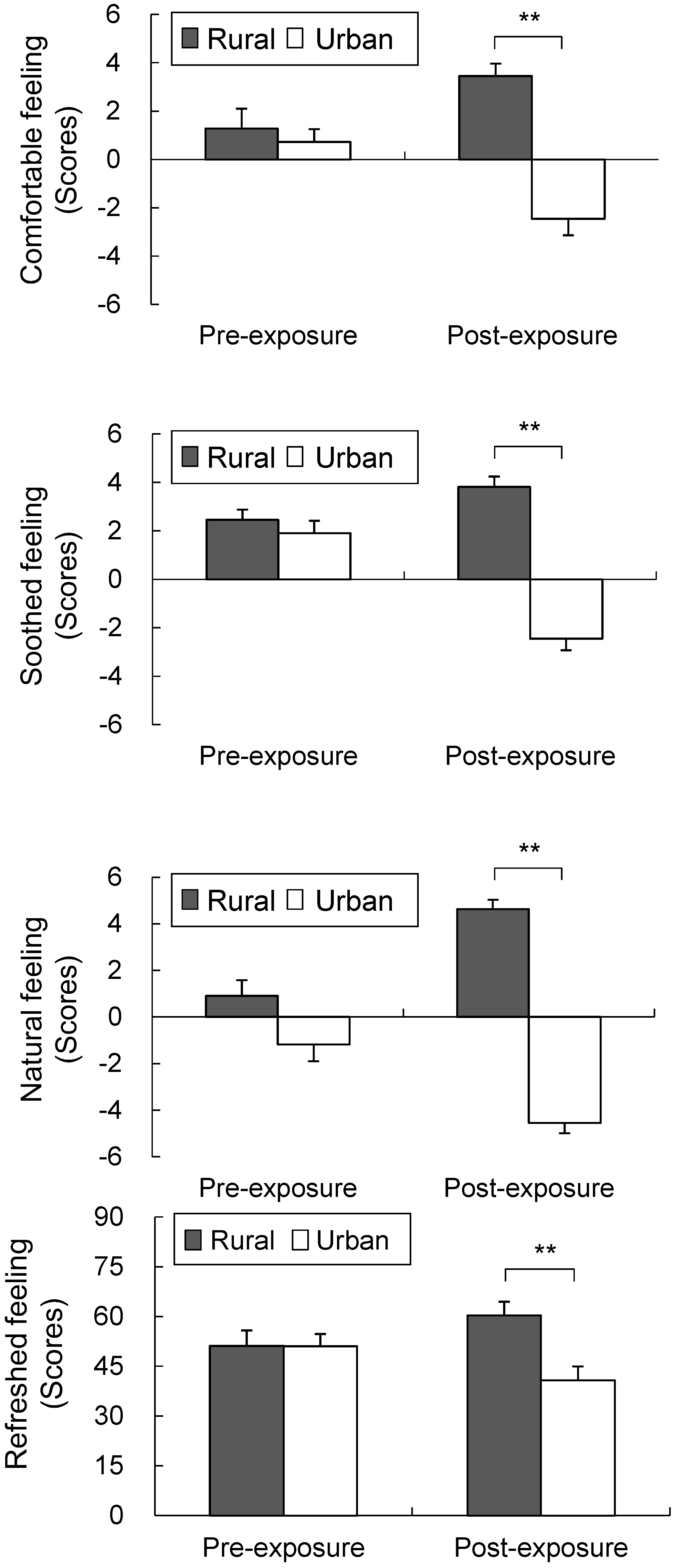
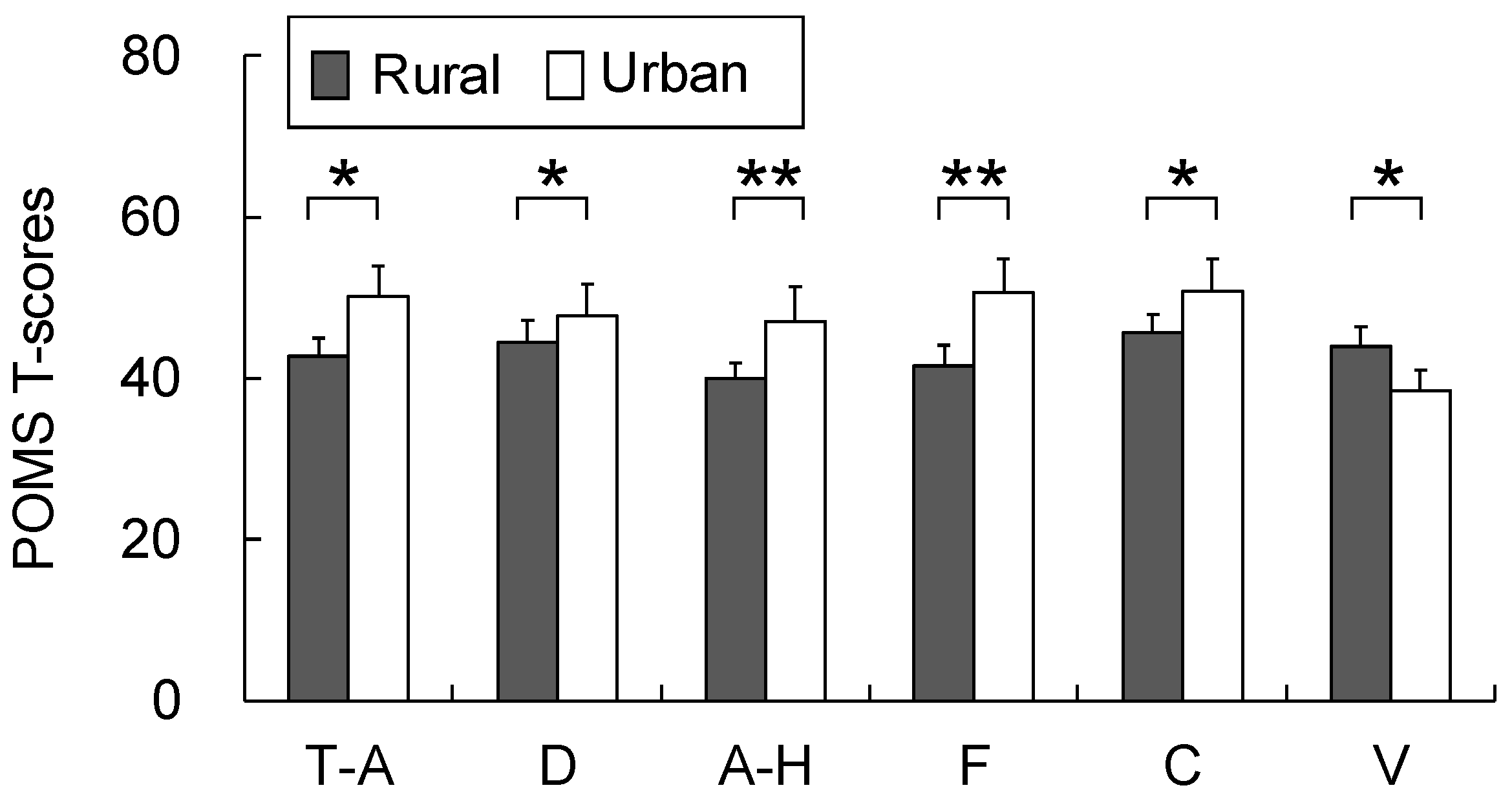
3.3. Air Quality Analysis
| A | |||
| Compounds | Concentrations (ng/m3) | ||
| Isoprene | 28.0 | ||
| Tricyclene | 13.1 | ||
| α-Pinene | 677.3 | ||
| Camphene | 107.7 | ||
| β-Pinene | 15.5 | ||
| Myrcene | 22.1 | ||
| δ-3-Carene | 16.7 | ||
| ρ-Cymene | 16.8 | ||
| D-Limonene | 53.4 | ||
| γ-Terpinene | 10.1 | ||
| α-Terpinolene | 3.7 | ||
| Bornyl acetate | 2.6 | ||
| These values are determined by absolute calibration method using toluene. | |||
| B | |||
| Groups | Compounds | Concentrations (ng/m3) | |
| Alkanes | n-Hexane | 3414 | |
| 2,4-Dimethylpentane | 421 | ||
| iso-Octane | 450 | ||
| Heptane | 678 | ||
| Octane | 188 | ||
| Nonane | 459 | ||
| Decane | 622 | ||
| Undecane | 344 | ||
| Dodecane | 42 | ||
| Tridecane | nd | ||
| Aromatic compounds | Benzene | 1481 | |
| Toluene | 14,104 | ||
| Ethylbenzene | 1771 | ||
| o, m, p-Xylene | 2873 | ||
| Styrene | 139 | ||
| m-Ethyltoluene | 1024 | ||
| p-Ethyltoluene | 424 | ||
| 1, 3, 5-Trimethylbenzene | 412 | ||
| o-Ethyltoluene | 374 | ||
| 1, 2, 4-Trimethylbenzene | 1870 | ||
| 1, 2, 3-Trimethylbenzene | 337 | ||
| 1, 2, 4, 5-Tetramethylbenzene | 35 | ||
| Terpenes | α-Pinene | 80 | |
| β-Pinene | 5 | ||
| D-Limonene | nd | ||
| Halogenated compounds | Dichloromethane | 5345 | |
| Chloroform | 1686 | ||
| 1, 2-Dichloroethane | 7 | ||
| Trichloroethylene | 16 | ||
| 1, 2-Dichloropropane | nd | ||
| Tetrachloroethylene | 165 | ||
| p-Dichlorobenzene | 294 | ||
| Esters | Ethyl acetate | 5722 | |
| Butyl acetate | 930 | ||
| Alcohols | Nonanol | 520 | |
| Decanol | 125 | ||
| Ethanol | nd | ||
| Aldehyde-ketone | Acetone | nd | |
| Methylethylketone | nd | ||
| Methylisobutylketone | 111 | ||
| These values are determined by absolute calibration method using toluene. | |||
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patel, R.B.; Burke, T.F. Urbanization—An emerging humanitarian disaster. N. Engl. J. Med. 2009, 361, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Antrop, M. Landscape change and the urbanization process in Europe. Landsc. Urban. Plan. 2004, 67, 9–26. [Google Scholar] [CrossRef]
- Mage, D.; Ozolins, G.; Peterson, P.; Webster, A.; Orthofer, R.; Vandeweerd, V.; Gwynne, M. Urban air pollution in megacities of the world. Atmos. Environ. 1996, 30, 681–686. [Google Scholar] [CrossRef]
- Kalnay, E.; Cai, M. Impact of urbanization and land-use change on climate. Nature 2003, 423, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J. The impact of the built environment on health: An emerging field. Amer. J. Public Health 2003, 93, 1382–1384. [Google Scholar] [CrossRef]
- Frumkin, H. Beyond toxicity: Human health and the natural environment. Amer. J. Prev. Med. 2001, 20, 234–240. [Google Scholar] [CrossRef]
- IUHPE. The Evidence of Health Promotion Effectiveness: Shaping Public Health in a New Europe; ECSC-EC-EAEC: Brussels, Belgium, 1999. [Google Scholar]
- Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M.; Meyer-Lindenberg, A. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 474, 498–501. [Google Scholar] [CrossRef] [PubMed]
- WHO. Urbanization and health. Bull. WHO 2010, 88, 245–246. [Google Scholar] [CrossRef]
- Adediran, O.S.; Adebayo, P.B.; Akintunde, A.A. Anthropometric differences among natives of Abuja living in urban and rural communities: Correlations with other cardiovascular risk factors. BMC Res. Notes 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Singh, G.M.; Paciorek, C.J.; Lin, J.K.; Cowan, M.J.; Finucane, M.M.; Farzadfar, F.; Stevens, G.A.; Riley, L.M.; Lu, Y.; et al. Global burden of metabolic risk factors of chronic diseases collaborating group: The global cardiovascular risk transition: Associations of four metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008. Circulation 2013, 127, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Delisle, H.; Ntandou-Bouzitou, G.; Agueh, V.; Sodjinou, R.; Fayomi, B. Urbanisation, nutrition transition and cardiometabolic risk: The Benin study. Brit. J. Nutr. 2012, 107, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Galea, S.; Uddin, M.; Koenen, K. The urban environment and mental disorders: Epigenetic links. Epigenetics 2011, 6, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Ginter, E.; Simko, V. Type 2 diabetes mellitus, pandemic in 21st century. Adv. Exp. Med. Biol. 2012, 771, 42–50. [Google Scholar] [PubMed]
- Gong, P.; Liang, S.; Carlton, E.J.; Jiang, Q.; Wu, J.; Wang, L.; Remais, J.V. Urbanisation and health in China. Lancet 2012, 379, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.H.; Brath, H. A global view on the development of non communicable diseases. Prev. Med. 2012, 54, S38–S41. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C.; Jacka, F.N. Nutritional psychiatry research: An emerging discipline and its intersection with global urbanization, environmental challenges and the evolutionary mismatch. J. Physiol. Anthropol. 2014, 33, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Li, Q.; Tyrväinen, L.; Tsunetsugu, Y.; Park, B.J.; Kagawa, T.; Miyazaki, Y. Nature therapy and preventive medicine. In Public Health–Social and Behavioral Health; Maddock, J., Ed.; Intech Online Publisher: Rijeka, Croatia, 2012; pp. 325–350. [Google Scholar]
- Hartig, T.; Mang, M.; Evans, G.W. Restorative effects of natural environment experience. Environ. Behav. 1991, 23, 3–26. [Google Scholar] [CrossRef]
- Herzog, T.R.; Black, A.M.; Fountaine, K.A.; Knotts, D.J. Reflection and attentional recovery as distinctive benefits of restorative environments. J. Environ. Psychol. 1997, 17, 165–170. [Google Scholar] [CrossRef]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Korpela, K.M.; Yién, M. Perceived health is associated with visiting natural favourite places in the vicinity. Health Place 2007, 13, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S. The influence of forest view through a window on job satisfaction and job stress. Scand. J. Forest Res. 2007, 22, 248–253. [Google Scholar] [CrossRef]
- Taylor, F.A.; Kuo, F.E.; Sullivan, W.C. Coping with ADD: The surprising connection to green play settings. Environ. Behav. 2001, 33, 54–77. [Google Scholar] [CrossRef]
- Ulrich, R.S. View through a window may influence recovery from surgery. Science 1984, 224, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol. 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Verderber, S. Dimensions of person-window transactions in the hospital environments. Environ. Behav. 1986, 18, 450–466. [Google Scholar] [CrossRef]
- Hartig, T.; Evans, G.W.; Jamner, L.D.; Davis, D.S.; Gärling, T. Tracking restoration in natural and urban field settings. J. Environ. Psychol. 2003, 23, 109–123. [Google Scholar] [CrossRef]
- Berman, M.G.; Jonides, J.; Kaplan, S. The cognitive benefits of interacting with nature. Psychol. Sci. 2008, 19, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Alcock, I.; White, W.P.; Wheeler, B.W.; Fleming, L.E.; Depledge, M.H. Longitudinal effects on mental health of moving to greener and less green urban areas. Environ. Sci. Technol. 2013, 48, 1247–1255. [Google Scholar] [CrossRef]
- Mitchell, R.; Popham, F. Effect of exposure to natural environment on health inequalities: An observational population study. Lancet 2008, 372, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Nakamura, K.; Watanabe, M. Urban residential environments and senior citizens’ longevity in megacity areas: The importance of walkable green spaces. J. Epidemiol. Community Health 2002, 56, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Hirano, H.; Kagawa, T.; Sato, M.; Miyazaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest)-using salivary cortisol and cerebral activity as indicators. J. Physiol. Anthropol. 2007, 26, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Kagawa, T.; Miyazaki, Y. Restorative effects of viewing real forest landscapes: Based on a comparison with urban landscapes. Scand. J. Forest Res. 2009, 24, 227–234. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Woo, J.M.; Kim, W.; Lim, S.K.; Chung, E.J. The effect of cognitive behavior therapy-based “Forest Therapy” program on blood pressure, salivary cortisol level, and quality of life in elderly hypertensive patients. Clin. Exp. Hypertens. 2012, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Park, B.J.; Ishii, H.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological effects of “Shinrin-yoku” (taking in the atmosphere of the forest) in an old-growth broadleaf forest in Yamagata prefecture, Japan. J. Physiol. Anthropol. 2007, 26, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Suzuki, H.; Miyazaki, Y.; Kagawa, T.; et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2007, 20, 3–8. [Google Scholar] [PubMed]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Li, Y.J.; Wakayama, Y.; et al. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J. Biol. Regul. Homeost. Agents. 2008, 22, 45–55. [Google Scholar] [PubMed]
- Rogge, E.; Nevens, F.; Gulinck, H. Perception of rural landscapes in Flanders: Looking beyond aesthetics. Landsc. Urban Plan 2007, 82, 159–174. [Google Scholar] [CrossRef]
- Fleischer, A.; Tsur, Y. The amenity value of agricultural landscape and rural-urban land allocation. J. Agric. Econ. 2008, 60, 132–153. [Google Scholar] [CrossRef]
- Lopez, R. Urban sprawl and risk for being overweight or obese. Amer. J. Public Health 2004, 94, 1574–1579. [Google Scholar] [CrossRef]
- McGranahan, D.A. Landscape influence on recent rural migration in the U.S. Landsc. Urban Plan 2008, 85, 228–240. [Google Scholar] [CrossRef]
- Kim, T.H.; Jeong, G.W.; Baek, H.S.; Kim, G.W.; Sundaram, T.; Kang, H.K.; Lee, S.W.; Kim, H.J.; Song, J.K. Human brain activation in response to visual stimulation and rural urban scenery pictures: A functional magnetic resonance imaging study. Sci. Total Environ. 2010, 408, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.; Aspinall, P. The restorative benefits of walking in urban and rural settings in adults with good and poor mental health. Health Place 2011, 17, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, S.; González-Roa, M.C.; Calatrava-Requena, J. Public preferences for landscape features: The case of agricultural landscape in mountainous Mediterranean areas. Land Use Policy 2009, 26, 334–344. [Google Scholar] [CrossRef]
- Harvey, T.; Works, M.A. Urban sprawl and rural landscape: Perceptions of landscape as amenity in Portland, Oregon. Local Environ. 2002, 7, 381–396. [Google Scholar] [CrossRef]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrväinen, L.; Kagawa, T.; et al. Influence of forest therapy on cardiovascular relaxation in young adults. Evid-Based Compl. Alt. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Groenewegen, P.G.; van den Berg, A.E.; de Vries, S.; Verheij, R.A. Vitamin G: Effects of green space on health, well-being, and social safety. BMC Public Health 2006, 6, 149. [Google Scholar] [CrossRef] [PubMed]
- Osgood, C.E.; Suci, G.J.; Tannenbaum, P.H. The Measurement of Meaning; University of Illinois Press: Urbana, IL, USA, 1957. [Google Scholar]
- Mackay, C.; Cox, T.; Burrows, G.; Lazzerini, T. An inventory for the measurement of self-reported stress and arousal. Br. J. Soc. Clin. Psychol. 1978, 17, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Araki, S.; Kawakami, N.; Takeshita, T. Production of the Japanese edition of Profile of Mood States (POMS): Assessment of reliability and validity. Jpn. J. Public Health 1990, 37, 913–918. [Google Scholar]
- Task Force of the European Society of Cardiology; the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Brown, D.K.; Barton, J.L.; Gladwell, V.F. Viewing nature scenes positively affects recovery of autonomic function following acute-mental stress. Environ. Sci. Technol. 2013, 47, 5562–5569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsunetsugu, Y.; Park, B.J.; Miyazaki, Y. Physiological effects of visual, olfactory, auditory, and tactile factors of forest environments. In Forest Medicine; Li, Q., Ed.; Nova Science Publisher: Hauppauge, NY, USA, 2012; pp. 169–181. [Google Scholar]
- Igarashi, M.; Yamamoto, T.; Lee, J.; Song, C.; Ikei, H.; Miyazaki, Y. Effects of stimulation by three-dimensional natural images on prefrontal cortex and autonomic nerve activity: A comparison with stimulation using two-dimensional images. Cogn. Process 2014, 15, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O. Biophilia: The Human Bond with Other Species; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Takayama, N.; Korpela, K.; Lee, J.; Morikawa, T.; Tsunetsugu, Y.; Park, B.J.; Li, Q.; Tyrväinen, L.; Miyazaki, Y.; Kagawa, T. Emotional, restorative and vitalizing effects of forest and urban environments at four sites in Japan. Int. J. Environ. Res. Public Health 2014, 11, 7207–7230. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, U. Psychoneuroendocrinoimmunology: The basis for a novel therapeutic approach in aging. Psychoneuroendocrinol 1992, 17, 411–420. [Google Scholar] [CrossRef]
- Goodkin, K.; Visser, A.P. (Eds.) Psychoneuroimmunology: Stress, Mental Disorders, and Health; American Psychiatric Press: Washington, DC, USA, 2000.
- Stilgoe, J.R. Gone barefoot lately? Amer. J. Prev. Med. 2001, 20, 243–244. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Lee, Y.; Park, B.J.; Tyrväinen, L.; Kagawa, T.; Miyazaki, Y. Physiological and psychological effects of viewing urban forest landscapes assessed by multiple measurements. Landsc. Urban Plan 2013, 113, 90–93. [Google Scholar] [CrossRef]
- Ernst, P.; Cormier, Y. Relative scarcity of asthma and atopy among rural adolescents raised on a farm. Am. J. Respir. Crit. Care Med. 2000, 161, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.D.; Taha, F. Global health benefits of being raised in a rural setting: Results from The National Comorbidity Survey. Psychiatr. Clin. Neurosci. 2014, 68, 395–403. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Park, B.-J.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Acute Effects of Exposure to a Traditional Rural Environment on Urban Dwellers: A Crossover Field Study in Terraced Farmland. Int. J. Environ. Res. Public Health 2015, 12, 1874-1893. https://doi.org/10.3390/ijerph120201874
Lee J, Park B-J, Ohira T, Kagawa T, Miyazaki Y. Acute Effects of Exposure to a Traditional Rural Environment on Urban Dwellers: A Crossover Field Study in Terraced Farmland. International Journal of Environmental Research and Public Health. 2015; 12(2):1874-1893. https://doi.org/10.3390/ijerph120201874
Chicago/Turabian StyleLee, Juyoung, Bum-Jin Park, Tatsuro Ohira, Takahide Kagawa, and Yoshifumi Miyazaki. 2015. "Acute Effects of Exposure to a Traditional Rural Environment on Urban Dwellers: A Crossover Field Study in Terraced Farmland" International Journal of Environmental Research and Public Health 12, no. 2: 1874-1893. https://doi.org/10.3390/ijerph120201874







