A Novel High-Throughput Approach to Measure Hydroxyl Radicals Induced by Airborne Particulate Matter
Abstract
:1. Introduction
2. Experimental Section
2.1. Molecular Probes and Reaction Products Detection
2.2. Molecular Probe Evaluation
2.3. PM-Induced Hydroxyl Radical Formation
2.3.1. Reaction Environment
2.3.2. Hydroxyl Radical Induced by Transition Metal Ions
2.3.3. Hydroxyl Radicals Induced by PM
3. Results
3.1. Limit of Detection (LOD)
3.2. Reactivity
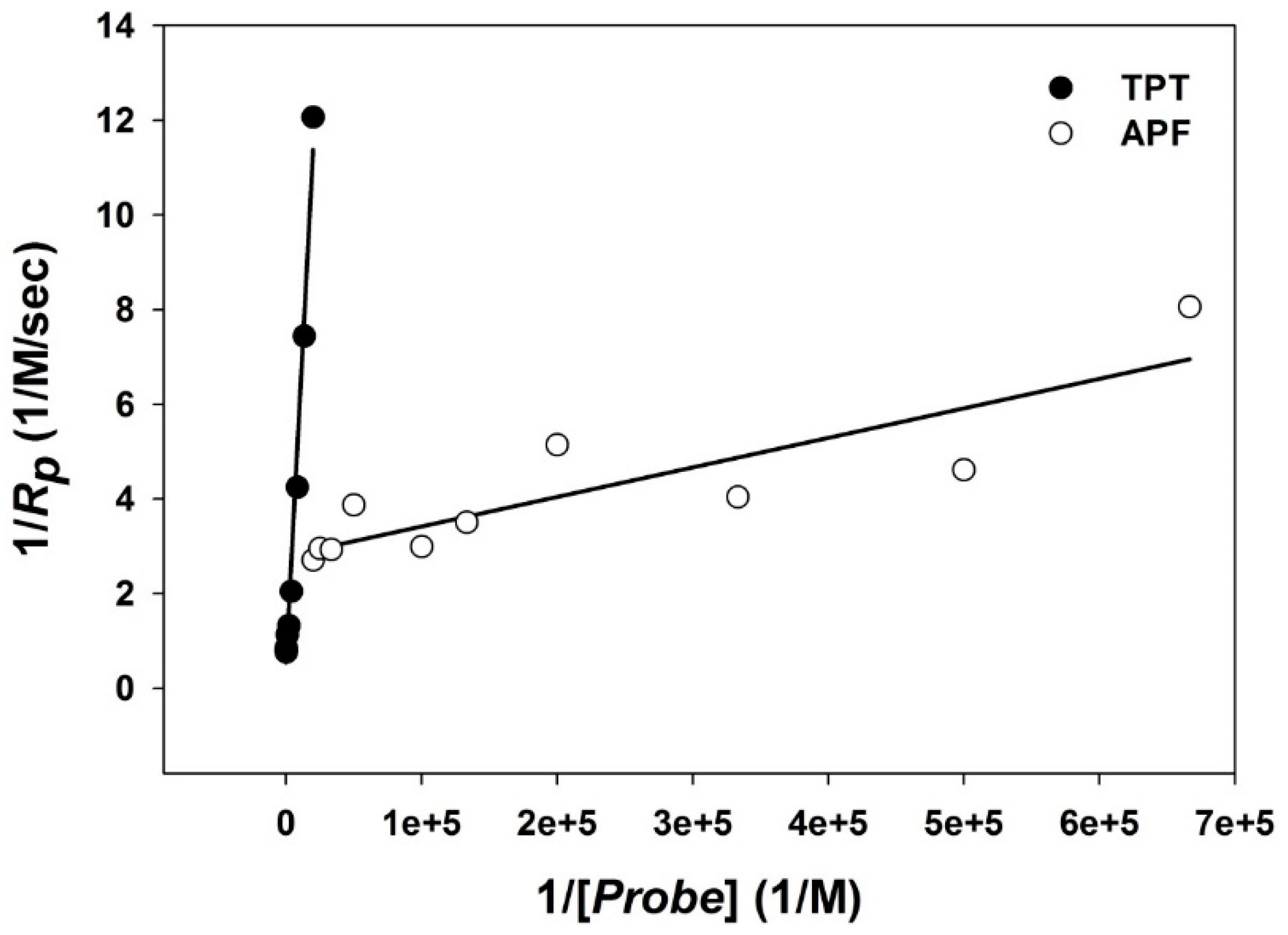
| kp (1/M/s) | TPT | APF | 3CCA | BA |
|---|---|---|---|---|
| Measured in our study | - | 2.9 × 1011 ± 8.5 × 1010 | 3.4 × 109 ± 2.2 × 106 | 3.5 × 109 ± 2.3 × 108 |
| Reported in the literature | a 3.3 × 109 | - | b 5.01 × 109 | c 3.0 × 109 |
3.3. Reaction Yield
3.4. PM-Induced •OH Formation
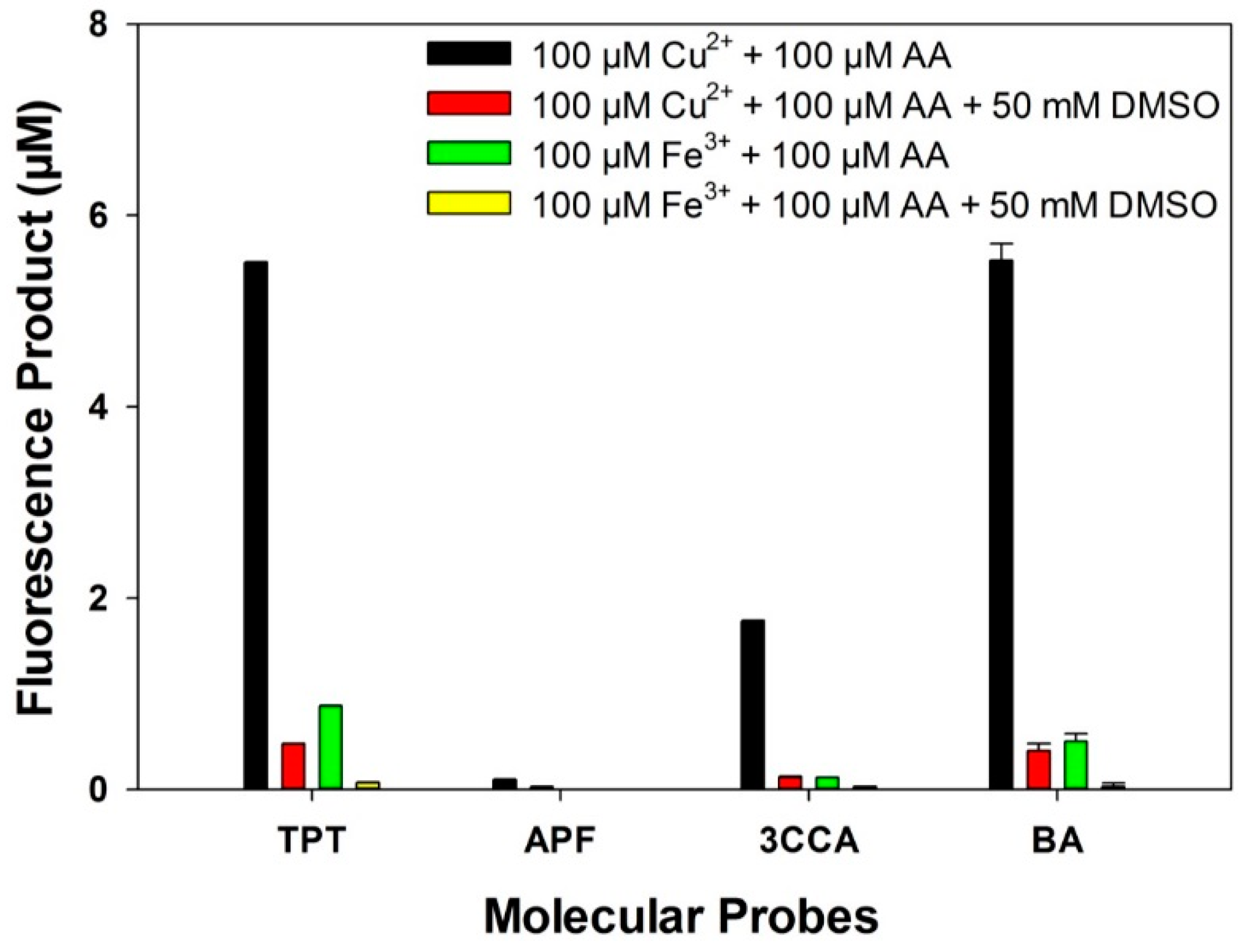
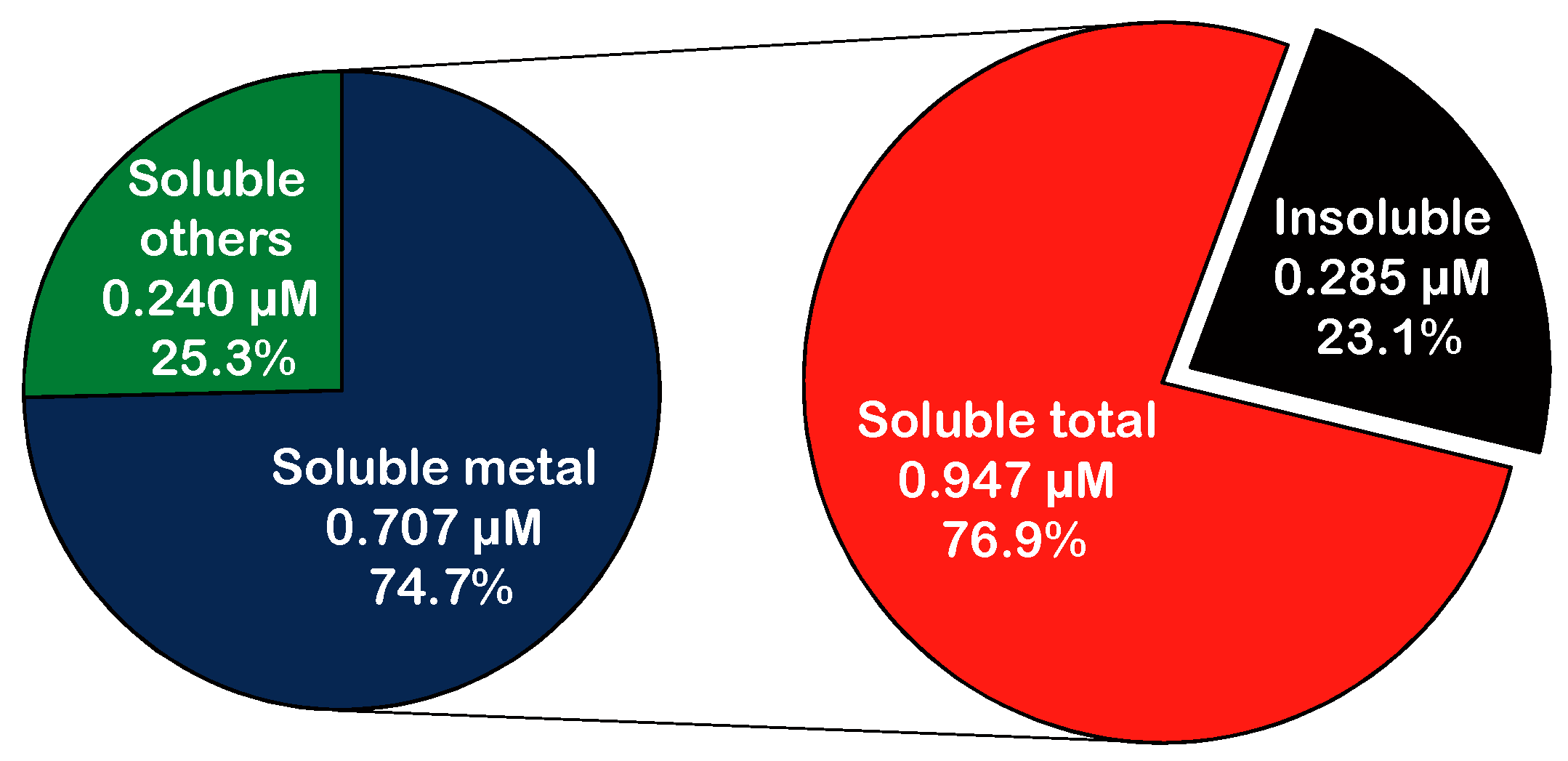
4. Discussion
4.1. Molecular Probe Selection for Ambient PM-Induced •OH
4.1.1. Limit of Detection (LOD)
4.1.2. Reactivity
4.1.3. Reaction Yield
4.1.4. Product Stability
4.1.5. The Solubility of a Molecular Probe
4.1.6. Fluorescence Intensity Affected by pH
4.1.7. A Brief Summary of the Molecular Probes Evaluated in the Study
| Molecular Probe Properties | Molecular Probe | |||
|---|---|---|---|---|
| TPT | APF | 3CCA | BA | |
| LOD (nM) | 17.59 | 0.1851 | 2.723 | 58.16 |
| Reactivity (1/M/s) | 3.3 × 109 a | 2.9 × 1011 | 3.4 × 109 | 3.5 × 109 |
| Yield (%) | 35 b | 1 | 11 | 35 |
| Product stability | High | Low | High | Low |
| Solubility | High | - | Low | High |
| Optimal pH range | 6~11 | >9 | >9 | 6~11 |
4.2. Hydroxyl Radicals Induced by Ambient PM
4.3. Strengths and Limitations
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- U.S. Environmental Protection Agency. Final Report: Integrated Science Assessment for Particulate Matter; EPA/600/R-08/139F; U.S. Environmental Protection Agency: Washington, DC, USA, 2009; pp. 122–137.
- Wittkopp, S.; Staimer, N.; Tjoa, T.; Stinchcombe, T.; Daher, N.; Schauer, J.J.; Shafer, M.M.; Sioutas, C.; Gillen, D.L.; Delfino, R.J. Nrf2-related gene expression and exposure to traffic-related air pollution in elderly subjects with cardiovascular disease: An exploratory panel study. J. Expo. Sci. Environ. Epidemiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Strak, M.; Janssen, N.A.H.; Godri, K.J.; Gosens, I.; Mudway, I.S.; Cassee, F.R.; Lebret, E.; Kelly, F.J.; Harrison, R.M.; Brunekreef, B.; et al. Respiratory health effects of airborne particulate matter: The role of particle size, composition, and oxidative potential-the raptes project. Environ. Health Perspect. 2012, 120, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Soppa, V.J.; Schins, R.P.; Hennig, F.; Hellack, B.; Quass, U.; Kaminski, H.; Kuhlbusch, T.A.; Hoffmann, B.; Weinmayr, G. Respiratory effects of fine and ultrafine particles from indoor sources—A randomized sham-controlled exposure study of healthy volunteers. Int. J. Environ. Res. Public Health 2014, 11, 6871–6889. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Pan, X.-C.; Kim, S.-Y.; Park, K.; Kim, Y.-H.; Kim, H.; Hong, Y.-C. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ. Health Perspect. 2010, 118, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Ciencewicki, J.; Trivedi, S.; Kleeberger, S.R. Oxidants and the pathogenesis of lung diseases. J. Allergy Clin. Immunol. 2008, 122, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Dick, C.A.; Brown, D.M.; Donaldson, K.; Stone, V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal. Toxicol. 2003, 15, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Weichenthal, S.A.; Godri-Pollitt, K.; Villeneuve, P.J. PM2.5, oxidant defence and cardiorespiratory health: A review. Environ. Health 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Ayres, J.G.; Borm, P.; Cassee, F.R.; Castranova, V.; Donaldson, K.; Ghio, A.; Harrison, R.M.; Hider, R.; Kelly, F.; Kooter, I.M.; et al. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential—A workshop report and consensus statement. Inhal. Toxicol. 2008, 20, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Gillen, D.L.; Schauer, J.J.; Shafer, M.M. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, M.; Bramble, L.A.; Schmitz, D.A.; Schauer, J.J.; Sioutas, C.; Harkema, J.R.; Nel, A.E. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ. Health Perspect. 2009, 117, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Duffin, R.; Borm, P.J.; Li, H.; Weishaupt, C.; Schins, R.P. Hydroxyl-radical-dependent DNA damage by ambient particulate matter from contrasting sampling locations. Environ. Res. 2006, 101, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In vitro toxicity of particulate matter (PM) collected at different sites in the netherlands is associated with pm composition, size fraction and oxidative potential—The raptes project. Part. Fibre Toxicol. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A.; Singh, M.; Eiguren-Fernandez, A.; Froines, J.R. Redox activity of airborne particulate matter at different sites in the los angeles basin. Environ. Res. 2005, 99, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Künzli, N.; Mudway, I.S.; Götschi, T.; Shi, T.; Kelly, F.J.; Cook, S.; Burney, P.; Forsberg, B.; Gauderman, J.W.; Hazenkamp, M.E.; et al. Comparison of oxidative properties, light absorbance, and total and elemental mass concentration of ambient PM 2.5 collected at 20 european sites. Environ. Health Perspect. 2006, 114, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Vidrio, E.; Jung, H.; Anastasio, C. Generation of hydroxyl radicals from dissolved transition metals in surrogate lung fluid solutions. Atmos. Environ. 2008, 42, 4369–4379. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, E.; Eiguren-Fernandez, A.; Delfino, R.J.; Sioutas, C.; Froines, J.R.; Cho, A.K. Determination of metal-based hydroxyl radical generating capacity of ambient and diesel exhaust particles. Inhal. Toxicol. 2009, 21, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Koide, S.; Taguchi, K.; Endo, A.; Nakai, Y.; Yoshikawa, T.; Shimojo, N. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 2002, 15, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Mudway, I.S.; Stenfors, N.; Duggan, S.T.; Roxborough, H.; Zielinski, H.; Marklund, S.L.; Blomberg, A.; Frew, A.J.; Sandström, T.; Kelly, F.J. An in vitro and in vivo investigation of the effects of diesel exhaust on human airway lining fluid antioxidants. Arch. Biochem. Biophys. 2004, 423, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Risom, L.; Møller, P.; Loft, S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res. 2005, 592, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.G.; Anastasio, C. Impacts of antioxidants on hydroxyl radical production from individual and mixed transition metals in a surrogate lung fluid. Atmos. Environ. 2011, 45, 7555–7562. [Google Scholar] [CrossRef] [PubMed]
- Cohn, C.A.; Pedigo, C.E.; Hylton, S.N.; Simon, S.R.; Schoonen, M.A. Evaluating the use of 3′-(p-aminophenyl) fluorescein for determining the formation of highly reactive oxygen species in particle suspensions. Geochem. Trans. 2009, 10. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Bakeas, E.; Vlahogianni, T. Electron paramagnetic resonance study of the generation of reactive oxygen species catalysed by transition metals and quinoid redox cycling by inhalable ambient particulate matter. Redox Rep. 2005, 10, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Schins, R.P.; Knaapen, A.M.; Kuhlbusch, T.; Pitz, M.; Heinrich, J.; Borm, P.J. Hydroxyl radical generation by electron paramagnetic resonance as a new method to monitor ambient particulate matter composition. J. Environ. Monit. 2003, 5, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Hellack, B.; Yang, A.; Cassee, F.R.; Janssen, N.A.; Schins, R.P.; Kuhlbusch, T.A. Intrinsic hydroxyl radical generation measurements directly from sampled filters as a metric for the oxidative potential of ambient particulate matter. J. Aerosol Sci. 2014, 72, 47–55. [Google Scholar] [CrossRef]
- Khan, N.; Wilmot, C.M.; Rosen, G.M.; Demidenko, E.; Sun, J.; Joseph, J.; O’Hara, J.; Kalyanaraman, B.; Swartz, H.M. Spin traps: In vitro toxicity and stability of radical adducts. Free Radic. Biol. Med. 2003, 34, 1473–1481. [Google Scholar] [CrossRef]
- Makino, K.; Hagiwara, T.; Hagi, A.; Nishi, M.; Murakami, A. Cautionary note for DMPO spin trapping in the presence of iron ion. Biochem. Biophys. Res. Commun. 1990, 172, 1073–1080. [Google Scholar] [CrossRef]
- Wei, R.; Oeser, T.; Billig, S.; Zimmermann, W. A high-throughput assay for enzymatic polyester hydrolysis activity by fluorimetric detection. Biotechnol. J. 2012, 7, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Linxiang, L.; Abe, Y.; Nagasawa, Y.; Kudo, R.; Usui, N.; Imai, K.; Mashino, T.; Mochizuki, M.; Miyata, N. An HPLC assay of hydroxyl radicals by the hydroxylation reaction of terephthalic acid. Biomed. Chromatogr. 2004, 18, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Saran, M.; Summer, K.H. Assaying for hydroxyl radicals: Hydroxylated terephthalate is a superior fluorescence marker than hydroxylated benzoate. Free Radic. Res. 1999, 31, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, G.; Maeyama, T.; Yamashita, S.; Taguchi, M.; Kimura, A.; Katsumura, Y.; Murakami, T. Determination of the time-dependent oh-yield by using fluorescent probe. Application to heavy ion irradiation. Chem. Phys. Lett. 2009, 468, 275–279. [Google Scholar] [CrossRef]
- Manevich, Y.; Held, K.D.; Biaglow, J.E. Coumarin-3-carboxylic acid as a detector for hydroxyl radicals generated chemically and by gamma radiation. Radiat. Res. 1997, 148, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Setsukinai, K.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [PubMed]
- Blackburn, A.C.; Doe, W.F.; Buffinton, G.D. Salicylate hydroxylation as an indicator of hydroxyl radical generation in dextran sulfate-induced colitis. Free Radic. Biol. Med. 1998, 25, 305–313. [Google Scholar] [CrossRef]
- Candeias, L.P.; Stratford, M.R.; Wardman, P. Formation of hydroxyl radicals on reaction of hypochlorous acid with ferrocyanide, a model iron(II) complex. Free Radic. Res. 1994, 20, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mishin, V.M.; Thomas, P.E. Characterization of hydroxyl radical formation by microsomal enzymes using a water-soluble trap, terephthalate. Biochem. Pharmacol. 2004, 68, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Mark, G.; Tauber, A.; Laupert, R.; Schuchmann, H.P.; Schulz, D.; Mues, A.; von Sonntag, C. OH-radical formation by ultrasound in aqueous solution—Part II: Terephthalate and fricke dosimetry and the influence of various conditions on the sonolytic yield. Ultrason. Sonochem. 1998, 5, 41–52. [Google Scholar] [CrossRef]
- Sun, G.; Crissman, K.; Norwood, J.; Richards, J.; Slade, R.; Hatch, G.E. Oxidative interactions of synthetic lung epithelial lining fluid with metal-containing particulate matter. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L807–L815. [Google Scholar] [PubMed]
- Zielinski, H.; Mudway, I.S.; Berube, K.A.; Murphy, S.; Richards, R.; Kelly, F.J. Modeling the interactions of particulates with epithelial lining fluid antioxidants. Am. J. Physiol. 1999, 277, L719–L726. [Google Scholar] [PubMed]
- Jung, H.; Guo, B.; Anastasio, C.; Kennedy, I.M. Quantitative measurements of the generation of hydroxyl radicals by soot particles in a surrogate lung fluid. Atmos. Environ. 2006, 40, 1043–1052. [Google Scholar] [CrossRef]
- Vidrio, E.; Phuah, C.H.; Dillner, A.M.; Anastasio, C. Generation of hydroxyl radicals from ambient fine particles in a surrogate lung fluid solution. Environ. Sci. Technol. 2009, 43, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.E.; van der Vliet, A.; O’Neill, C.A.; Louie, S.; Halliwell, B. Oxidants, antioxidants, and respiratory tract lining fluids. Environ. Health Perspect. 1994, 102, S185–S191. [Google Scholar] [CrossRef]
- Van der Vliet, A.; O’Neill, C.A.; Cross, C.E.; Koostra, J.M.; Volz, W.G.; Halliwell, B.; Louie, S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol. 1999, 276, L289–L296. [Google Scholar] [PubMed]
- Leinonen, H.; Lehto, J. Ion-exchange of nickel by iminodiacetic acid chelating resin chelex 100. React. Funct. Polym. 2000, 43, 1–6. [Google Scholar] [CrossRef]
- Donaldson, K.; Beswick, P.H.; Gilmour, P.S. Free radical activity associated with the surface of particles: A unifying factor in determining biological activity? Toxicol. Lett. 1996, 88, 293–298. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. AirData. Available online: http://www3.epa.gov/airquality/airdata/index.html (accessed on 23 October 2015).
- U.S. Environmental Protection Agency. Highlights of the Exposure Factors Handbook (Final Report); EPA/600/R-10/030; U.S. Environmental Protection Agency: Washington, DC, USA, 2011; pp. 15–18.
- Balásházy, I.; Hofmann, W.; Heistracher, T. Local particle deposition patterns may play a key role in the development of lung cancer. J. Appl. Physiol. 2003, 94, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Phalen, R.F.; Oldham, M.J.; Nel, A.E. Tracheobronchial particle dose considerations for in vitro toxicology studies. Toxicol. Sci. 2006, 92, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.M.; Roberts, C.M.; Bryant, D.H.; Cairns, D.; Yeates, M.; Morgan, G.W.; Martin, B.J.; Blake, H.; Penny, R.; Zaunders, J.J.; et al. Smoking-induced changes in epithelial lining fluid volume, cell density and protein. Eur. Respir. J. 1992, 5, 780–784. [Google Scholar] [PubMed]
- Rennard, S.I.; Basset, G.; Lecossier, D.; O’Donnell, K.M.; Pinkston, P.; Martin, P.G.; Crystal, R.G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 1986, 60, 532–538. [Google Scholar] [PubMed]
- Balásházy, I.; Hofmann, W.; Heistracher, T. Computation of local enhancement factors for the quantification of particle deposition patterns in airway bifurcations. J. Aerosol Sci. 1999, 30, 185–203. [Google Scholar] [CrossRef]
- Bolch, W.E.; Farfán, E.B.; Huh, C.; Huston, T.E.; Bolch, W.E. Influences of parameter uncertainties within the ICRP 66 respiratory tract model: Particle deposition. Health Phys. 2001, 81, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Page, S.E.; Arnold, W.A.; McNeill, K. Terephthalate as a probe for photochemically generated hydroxyl radical. J. Environ. Monit. 2010, 12, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.W. The radiation chemistry of the terephthalate dosimeter. Radiat. Res. 1980, 83, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Oturan, M.A.; Pinson, J. Hydroxylation by electrochemically generated OH.bul. radicals. Mono- and polyhydroxylation of benzoic acid: Products and isomer distribution. J. Phys. Chem. 1995, 99, 13948–13954. [Google Scholar] [CrossRef]
- Zhou, X.; Mopper, K. Determination of photochemically produced hydroxyl radicals in seawater and freshwater. Mar. Chem. 1990, 30, 71–88. [Google Scholar] [CrossRef]
- Barreto, J.C.; Smith, G.S.; Strobel, N.H.; McQuillin, P.A.; Miller, T.A. Terephthalic acid: A dosimeter for the detection of hydroxyl radicals in vitro. Life Sci. 1995, 56, PL89–PL96. [Google Scholar] [CrossRef]
- Fang, X.; Mark, G.; von Sonntag, C. OH radical formation by ultrasound in aqueous solutions part I: The chemistry underlying the terephthalate dosimeter. Ultrason. Sonochem. 1996, 3, 57–63. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.R.; Robins, E. Fluorescence of substituted 7-hydroxycoumarins. Anal. Chem. 1968, 40, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Ellingboe, J.; Runnels, J. Solubilities of disodium terephthalate in aqueous solutions of sodium carbonate and sodium bicarbonate. J. Chem. Eng. Data 1966, 11, 185–187. [Google Scholar] [CrossRef]
- Jouyban, A. Handbook of Solubility Data for Pharmaceuticals; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Buettner, G.R.; Doherty, T.P.; Patterson, L.K. The kinetics of the reaction of superoxide radical with Fe (III) complexes of EDTA, DETAPAC and HEDTA. FEBS Lett. 1983, 158, 143–146. [Google Scholar] [CrossRef]
- Shen, H.; Anastasio, C. Formation of hydroxyl radical from san joaquin valley particles extracted in a cell-free surrogate lung fluid. Atmos. Chem. Phys. 2011, 11, 9671–9682. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Barakat, A.; Anastasio, C. Generation of hydrogen peroxide from San Joaquin Valley particles in a cell-free solution. Atmos. Chem. Phys. 2011, 11, 753–765. [Google Scholar] [CrossRef]
- Hu, S.; Polidori, A.; Arhami, M.; Shafer, M.; Schauer, J.; Cho, A.; Sioutas, C. Redox activity and chemical speciation of size fractioned PM in the communities of the Los Angeles-Long beach harbor. Atmos. Chem. Phys. 2008, 8, 6439–6451. [Google Scholar] [CrossRef]
- Shafer, M.M.; Perkins, D.A.; Antkiewicz, D.S.; Stone, E.A.; Quraishi, T.A.; Schauer, J.J. Reactive oxygen species activity and chemical speciation of size-fractionated atmospheric particulate matter from Lahore, Pakistan: An important role for transition metals. J. Environ. Monit. 2010, 12, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Polidori, A.; Schauer, J.J.; Shafer, M.M.; Cassee, F.R.; Sioutas, C. Physicochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 southern California wildfires. Environ. Sci. Technol. 2009, 43, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Diurnal trends in oxidative potential of coarse particulate matter in the Los Angeles Basin and their relation to sources and chemical composition. Environ. Sci. Technol. 2012, 46, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.; Mishin, V.; Welsh, W.; Lu, S.-E.; Laskin, J.D.; Kipen, H.; Meng, Q. A Novel High-Throughput Approach to Measure Hydroxyl Radicals Induced by Airborne Particulate Matter. Int. J. Environ. Res. Public Health 2015, 12, 13678-13695. https://doi.org/10.3390/ijerph121113678
Son Y, Mishin V, Welsh W, Lu S-E, Laskin JD, Kipen H, Meng Q. A Novel High-Throughput Approach to Measure Hydroxyl Radicals Induced by Airborne Particulate Matter. International Journal of Environmental Research and Public Health. 2015; 12(11):13678-13695. https://doi.org/10.3390/ijerph121113678
Chicago/Turabian StyleSon, Yeongkwon, Vladimir Mishin, William Welsh, Shou-En Lu, Jeffrey D. Laskin, Howard Kipen, and Qingyu Meng. 2015. "A Novel High-Throughput Approach to Measure Hydroxyl Radicals Induced by Airborne Particulate Matter" International Journal of Environmental Research and Public Health 12, no. 11: 13678-13695. https://doi.org/10.3390/ijerph121113678






