Lower Risk of Stroke after Deformity Surgery: Long Term Benefit Demonstrated by a National Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database, Registrations, and Standard Protocol Approvals
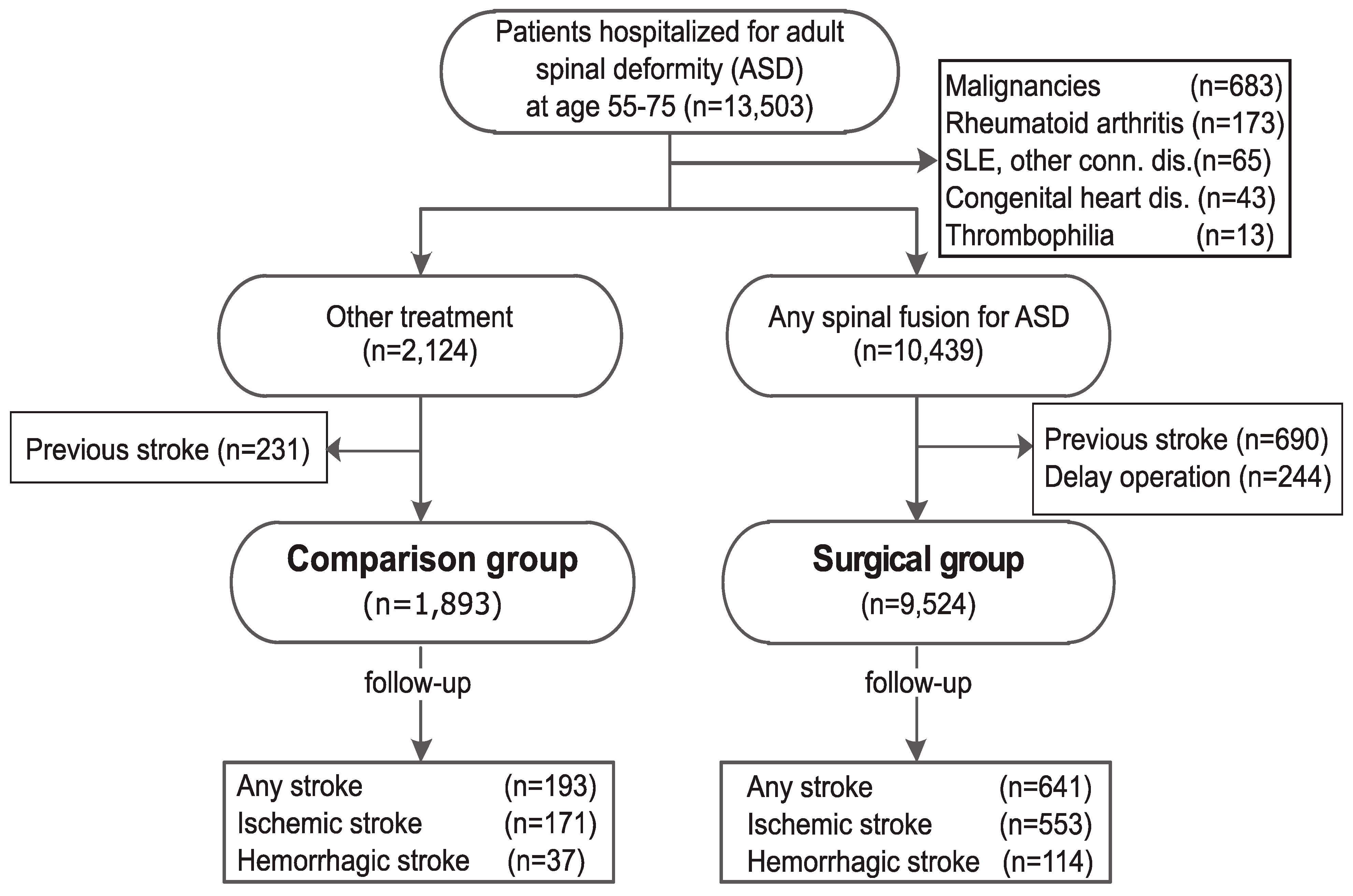
2.2. Hospitalization for ASD and Spinal Fusion Surgery
2.3. Surgical vs. Control Group
2.4. Covariates and Outcomes
2.5. Statistical Analysis
3. Results
Incidence of Stroke in ASD Patients
| Full Cohort (Original Unmatched Cohort) | Propensity Score Matched Cohort (Ratio 1:1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparison Group | Surgical Group | p-Value | Comparison Group | Surgical Group | p-Value | |||||
| n = 1983 | (%) | n = 9524 | (%) | n = 1872 | (%) | n = 1872 | (%) | |||
| Demographic characteristics | ||||||||||
| Gender | <0.001 | 0.475 | ||||||||
| Female | 1193 | (60.2) | 6480 | (68.0) | 1184 | (63.2) | 1205 | (64.4) | ||
| Male | 700 | (35.3) | 3044 | (32.0) | 688 | (36.8) | 667 | (35.6) | ||
| Age, Mean (SD) | 67 | (6.0) | 66.4 | (5.7) | <0.001 | 67.0 | (6.0) | 67.3 | (5.5) | 0.083 |
| Charlson’s score (SD) | 1.1 | (1.5) | 0.7 | (1.3) | <0.001 | 1.0 | (1.4) | 1.0 | (1.5) | 0.732 |
| Comorbidities | ||||||||||
| Hypertension | 0.879 | 0.534 | ||||||||
| Yes | 635 | (32.0) | 3212 | (33.7) | 622 | (33.2) | 640 | (34.2) | ||
| No | 1258 | (63.4) | 6312 | (66.3) | 1250 | (66.8) | 1232 | (65.8) | ||
| Diabetes | 0.402 | 0.965 | ||||||||
| Yes | 318 | (16.0) | 1526 | (16.0) | 308 | (16.5) | 309 | (16.5) | ||
| No | 1575 | (79.4) | 7998 | (84.0) | 1564 | (83.5) | 1563 | (83.5) | ||
| Arrythmia | <0.001 | 0.698 | ||||||||
| Yes | 144 | (7.3) | 496 | (5.2) | 131 | (7.0) | 125 | (6.7) | ||
| No | 1749 | (88.2) | 9028 | (94.8) | 1741 | (93.0) | 1747 | (93.3) | ||
| Coronary heart disease | <0.001 | 0.795 | ||||||||
| Yes | 333 | (16.8) | 1065 | (11.2) | 318 | (17.0) | 324 | (17.3) | ||
| No | 1560 | (78.7) | 8459 | (88.8) | 1554 | (83.0) | 1548 | (82.7) | ||
| Dyslipidemia | <0.001 | 0.812 | ||||||||
| Yes | 163 | (8.2) | 590 | (6.2) | 153 | (8.2) | 157 | (8.4) | ||
| No | 1730 | (87.2) | 8934 | (93.8) | 1719 | (91.8) | 1715 | (91.6) | ||
| Outcome | ||||||||||
| Any stroke | 193 | (9.7) | 641 | (6.7) | <0.001 | 191 | (10.2) | 132 | (7.1) | <0.001 |
| Ischemic stroke | 171 | (8.6) | 553 | (5.8) | <0.001 | 169 | (9.0) | 117 | (6.3) | 0.001 |
| Hemorrhagic stroke | 37 | (1.9) | 114 | (1.2) | 0.008 | 37 | (2.0) | 24 | (1.3) | 0.093 |
| Total Sample (Per 1000 Person-Year) | Comparison Group (Per 1000 Person-Year) | Surgical Group (Per 1000 Person-Year) | ||
|---|---|---|---|---|
| Incidence of hospitalized for stroke | 16.53 | 20.89 | 15.55 | |
| Number of hospitalized for stroke | 834 | 193 | 641 | |
| Observed person-years | 50,450.10 | 9240.80 | 41,209.30 | |
| Crude hazard ratio (95% C.I.) | 1.34 | 1.00 | (1.14–1.58) *** | |
| Adjusted hazard ratio, full cohort (95% C.I.) a | 1.19 | 1.00 | (1.01–1.39) * | |
| Adjusted hazard ratio, propensity score matched cohort (95% C.I.) b | 1.28 | 1.00 | (1.02–1.60) * |

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dorward, I.G.; Lenke, L.G. Osteotomies in the posterior-only treatment of complex adult spinal deformity: A comparative review. Neurosurg. Focus 2010, 28. [Google Scholar] [CrossRef] [PubMed]
- Lenke, L.G.; Sides, B.A.; Koester, L.A.; Hensley, M.; Blanke, K.M. Vertebral column resection for the treatment of severe spinal deformity. Clin. Orthop. Relat. Res. 2010, 468, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, C.D.; Starr, P.A.; Mummaneni, P.V. Spinal deformity and Parkinson disease: A treatment algorithm. Neurosurg. Focus 2010, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Mummaneni, P.V. Minimally invasive surgery for thoracolumbar spinal deformity: Initial clinical experience with clinical and radiographic outcomes. Neurosurg. Focus 2010, 28. [Google Scholar] [CrossRef] [PubMed]
- Yadla, S.; Maltenfort, M.G.; Ratliff, J.K.; Harrop, J.S. Adult scoliosis surgery outcomes: A systematic review. Neurosurg. Focus 2010, 28. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Boerma, T.; Ma Fat, D. Global and regional causes of death. Brit. Med. Bull. 2009, 92, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, X.; Huang, S.; Zhu, Z.; Qiao, J.; Zhu, F.; Mao, S.; Ding, Y.; Qiu, Y. Degenerative lumbar scoliosis in Chinese Han population: Prevalence and relationship to age, gender, bone mineral density, and body mass index. Eur. Spine J. 2013, 22, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; King, A.B.; Boachie-Adjei, O. Characterization of osteopenia/osteoporosis in adult scoliosis: Does bone density affect surgical outcome? Spine 2011, 36, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Wu, J.C.; Huang, W.C.; Wu, H.T.; Chiou, H.J.; Liu, L.; Chen, Y.C.; Chen, T.J.; Cheng, H.; Chang, C.Y.; et al. The risk of stroke after percutaneous vertebroplasty for osteoporosis: A population-based cohort study. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Chen, Y.C.; Liu, L.; Chen, T.J.; Huang, W.C.; Thien, P.F.; Cheng, H.; Lo, S.S. The risk of stroke after spinal fusion surgery: A national cohort study. Spine J. 2012, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Chen, Y.C.; Liu, L.; Huang, W.C.; Thien, P.F.; Chen, T.J.; Cheng, H.; Lo, S.S. Lumbar spine fusion surgery and stroke: A national cohort study. Eur. Spine J. 2012, 21, 2680–2687. [Google Scholar] [CrossRef]
- Lee, I.M.; Paffenbarger, R.S., Jr. Physical activity and stroke incidence: The Harvard alumni health study. Stroke 1998, 29, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, G.; Shaper, A.G. Physical activity and stroke in British middle aged men. BMJ 1992, 304, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Wendel-Vos, G.C.; Schuit, A.J.; Feskens, E.J.; Boshuizen, H.C.; Verschuren, W.M.; Saris, W.H.; Kromhout, D. Physical activity and stroke. A meta-analysis of observational data. Int. J. Epidemiol. 2004, 33, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.D.; Folsom, A.R.; Blair, S.N. Physical activity and stroke risk: A meta-analysis. Stroke 2003, 34, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Colditz, G.A.; Ascherio, A.; Rexrode, K.M.; Willett, W.C.; Manson, J.E. Physical activity and risk of stroke in women. JAMA 2000, 283, 2961–2967. [Google Scholar] [CrossRef] [PubMed]
- Sattelmair, J.R.; Kurth, T.; Buring, J.E.; Lee, I.M. Physical activity and risk of stroke in women. Stroke 2010, 41, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Agnarsson, U.; Thorgeirsson, G.; Sigvaldason, H.; Sigfusson, N. Effects of leisure-time physical activity and ventilatory function on risk for stroke in men: The Reykjavik study. Ann. Intern. Med. 1999, 130, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Sarti, C.; Jousilahti, P.; Silventoinen, K.; Barengo, N.C.; Tuomilehto, J. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke 2005, 36, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B.; Adams, R.; Alberts, M.J.; Appel, L.J.; Brass, L.M.; Bushnell, C.D.; Culebras, A.; Degraba, T.J.; Gorelick, P.B.; Guyton, J.R.; et al. Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006, 37, 1583–1633. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B.; Bushnell, C.D.; Adams, R.J.; Appel, L.J.; Braun, L.T.; Chaturvedi, S.; Creager, M.A.; Culebras, A.; Eckel, R.H.; Hart, R.G.; et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 517–584. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-C.; Chung, W.-F.; Liu, S.-W.; Chang, P.-Y.; Chen, L.-F.; Wu, J.-C.; Chen, Y.-C.; Huang, W.-C.; Liu, L.; Cheng, H.; et al. Lower Risk of Stroke after Deformity Surgery: Long Term Benefit Demonstrated by a National Cohort Study. Int. J. Environ. Res. Public Health 2015, 12, 12618-12627. https://doi.org/10.3390/ijerph121012618
Huang L-C, Chung W-F, Liu S-W, Chang P-Y, Chen L-F, Wu J-C, Chen Y-C, Huang W-C, Liu L, Cheng H, et al. Lower Risk of Stroke after Deformity Surgery: Long Term Benefit Demonstrated by a National Cohort Study. International Journal of Environmental Research and Public Health. 2015; 12(10):12618-12627. https://doi.org/10.3390/ijerph121012618
Chicago/Turabian StyleHuang, Liang-Chung, Wu-Fu Chung, Shih-Wei Liu, Peng-Yuan Chang, Li-Fu Chen, Jau-Ching Wu, Yu-Chun Chen, Wen-Cheng Huang, Laura Liu, Henrich Cheng, and et al. 2015. "Lower Risk of Stroke after Deformity Surgery: Long Term Benefit Demonstrated by a National Cohort Study" International Journal of Environmental Research and Public Health 12, no. 10: 12618-12627. https://doi.org/10.3390/ijerph121012618






