1. Introduction
Sex and gender, which can be operationalized as biology and culture, have the potential to confound results in environmental health studies of air pollution. The terms “sex” and “gender” have often been used interchangeably to simply differentiate men and women, but furthermore Clougherty [
1] suggests that study design or analytical approaches limit the extrication of sex and gender effects in studies where different meanings are assigned. Regarding studies on health effects of air pollution general, residence-based pollutant exposure models are limited by lack of temporal variability associated with daily activities, while longitudinal studies are often limited by spatially aggregated measures of exposure. Even less is understood about how gender as a cultural construct interacts with these limitations, therefore physiological systems have hitherto provided the most likely sources of observed differences in effects of pollution on males and females.
Effects of criteria air contaminants on the respiratory system are well documented, and several studies have reported differing outcomes for males and females [
2,
3]. Meta-analyses of respiratory effect modification of sex and gender are difficult to complete due to varying exposure mixes, outcomes and analytic techniques, however, more studies report stronger effects among women [
1]. Causal mechanisms for this remain unclear though experimental and clinical studies have corroborated this assertion and offer at least a partial explanation. For example, lung particulate matter deposition characteristics differ among men and women and the relative amount of deposition is greater in women due to higher flow rates [
4]. As measured by fatigue and pulmonary function, women are slightly more sensitive than men to effects of 2-propanol and
m-xylene vapours, which are among volatile organic compounds (VOCs) released by petrochemical processing and combustion engine exhaust [
5].
Cyclical fluctuations in sex hormones during the reproductive period of life increases the prevalence rates of atopic disease in women, though rates are lower compared to males during childhood and after menopause [
6]. Females have a lower risk of developing asthma in their childhood, equal risk during adolescence, and higher risk during early adulthood, which is attributed to smaller airway caliber and hormonal factors [
7], while air pollution is known to exacerbate asthma [
8]. Interestingly, immunoglobulin E (IgE) serum levels are higher in males throughout life, but sex specific differences in allergen sensitivity are inconsistent [
6]. Air pollutants can trigger IgE responses [
9], and a population-based study in London, England observed increased primary care consultations for allergic rhinitis in association with outdoor air pollution [
10].
However, sex and gender differences in susceptibility to air pollution are expressed beyond the respiratory system. Transdermal exposure pathways along with the irritant properties of pollution upon the skin itself are important as women exhibit higher rates of skin disease [
11]. There is also evidence in support of hormonal status attenuating the effect of particulate matter on heart disease in women less than 60 years of age [
12]. Complementing our understanding of biologically plausible mechanisms are environmental health studies that demonstrate significant associations between air pollution exposure profiles and sociocultural, socioeconomic and demographic factors [
13,
14], all of which can be influenced by gender. Furthermore, stress can potentiate or attenuate impacts of air pollution, and both hormonal and sociocultural characteristics of sex and gender, respectively, can moderate stress levels [
15].
Taken together, the aforementioned and broad range of outcomes and moderating factors associated with air pollution effects on health produce a complex challenge to public health policy makers, practitioners and administrators. As previously mentioned, both study design and analytical approaches are important considerations when attempting to disentangle the closely related, but distinct constructs of sex and gender. Therefore, this study used a novel analytical approach to determine the influence of sex and gender on susceptibility to short-term effects of air pollution. Recursive partitioning and regression modeling were utilized to examine the characteristics of sex and gender that contribute to the commonly observed stronger effect of air pollution on women.
5. Discussion
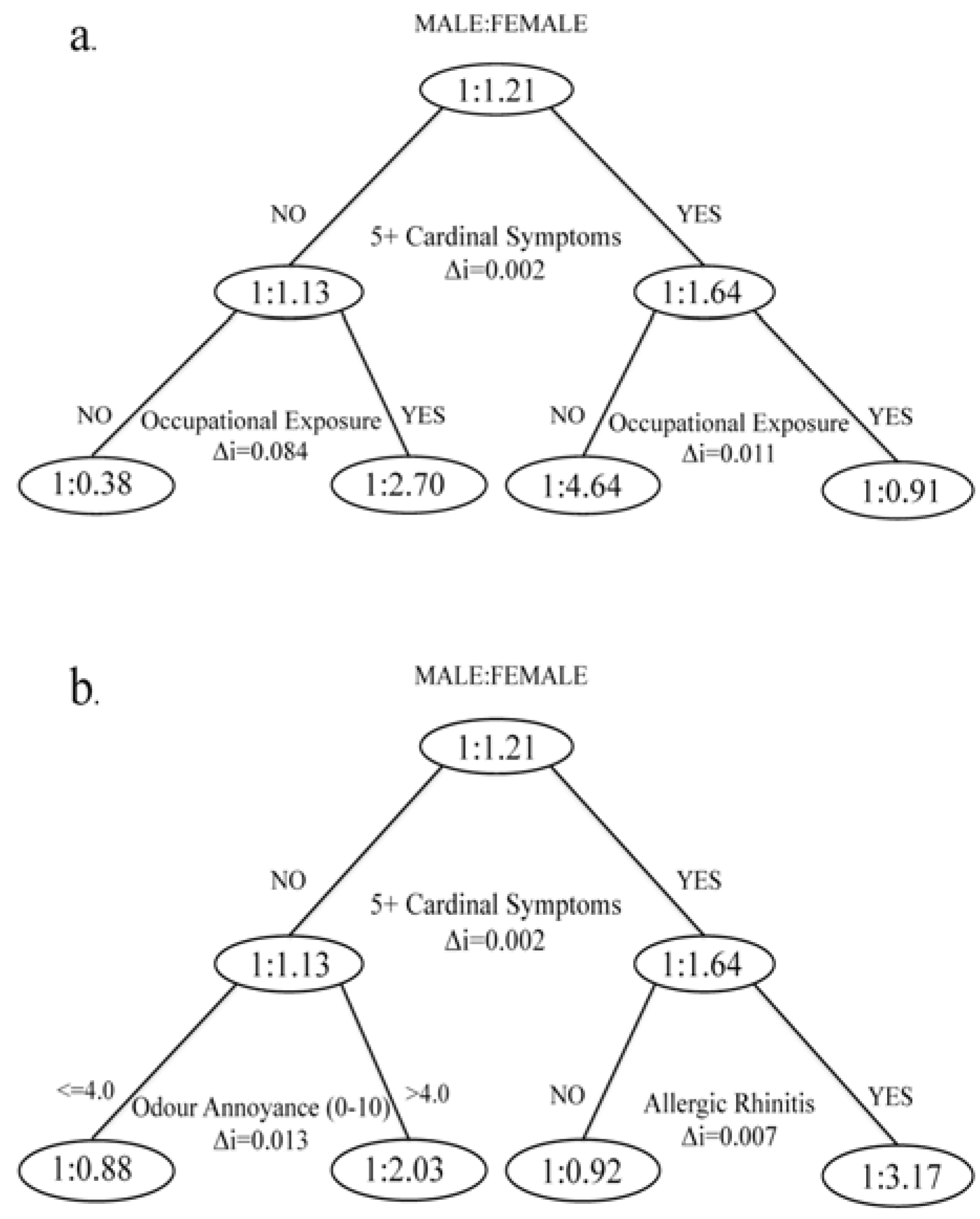
Overall, the study confirmed previous findings that demonstrated the effects of demographics, SES and health covariates on short-term effects of air pollution. We observed that women were approximately 50% more likely than men to report five or more cardinal symptoms of exposure to a combination of NO2, SO2, and BTEX. There is, however, an important distinction between this study and other work on short-term pollution effects. Our outcome measurement provided a much broader indication of potential pollution health effects than studies looking at mortality or hospitalization, although we also observed elevated probabilities of reporting cardinal symptoms by those traditionally thought to be at higher risk. The analysis showed that both biological and cultural differences between men and women were influential in predicting symptoms of pollution exposure.
We found that females had an increased susceptibility to 5+ CS reporting when they were also dealing with allergic diseases. Females had higher rates of allergic disease in our sample. Although working with female adolescents, Fagan and others also reported higher rates of allergies among females [
31]. Previous findings s on the susceptibility to air pollution among allergy sufferers remain equivocal [
10,
32], though children with allergies can have more severe asthma due to air pollution [
33]. There are genetic differences between sexes that influence inflammatory responses to allergens [
34], while sex hormones can affect the immune system and cause chemical hypersensitivity [
35]. These findings provide biological plausibility for the potentiation of air pollution effects in females being due to allergic disease.
Women in our study also had a significantly higher rate of skin conditions, and the prevalence of eczema is associated with air pollution even in areas with relatively low concentrations [
36]. Previous research found that females report skin conditions such as hand eczema more often, and most female-dominated occupations require wet work and are more likely to cause irritant contact dermatitis [
11]. Kreutzer
et al. [
37] found that females reported chemical sensitivity more often, but also that both males and females believed they were made sick by common chemical exposures. The possibility for psychosomatic effects therefore exists, but more importantly our findings support previous research that found women to (correctly) perceive air pollution as a higher risk to their health than men [
38]. Taken together these findings suggest that complex interactions between gendered co-exposures at work, sex-related determinants of vulnerability and ambient pollution exposure profiles can confound health effects of air pollution.
We found that occupational exposure modified the associations between chronic diseases and cardinal symptom reporting. Specifically, skin conditions and kidney diseases were a strong predictor of 5+ CS in the sub-sample with no occupational exposure
versus hypertension having a strong effect among occupationally exposed. Interestingly, certain kidney diseases and atopic disorders are associated [
39]. The difficulty of determining the moderating effects of occupational co-exposure was illustrated by a re-analysis of data for The Six Cities Study of mortality associated with PM
2.5, which found that the effect of air pollution was not consistently different in individuals with “dirty jobs” and exposure to lung carcinogens at work [
40]. We found that the effects of air pollution were non-significant among people who reported occupational exposure and highly significant among respondents with no occupational exposure. This does not, however, suggest that people with occupational exposure are less susceptible to effects of air pollution, but rather highlights the potentially confounding effects of aggregate exposure assessment and the importance of controlling for co-exposures in industrial cities like Sarnia [
16].
The relationship between hypertension and cardinal symptom reporting as shown in our analysis is complicated because air pollution can cause both hypertension [
41] and cardinal symptoms independently; several of our outcome indicators, including nausea and trouble breathing, are also side effects of antihypertensive drugs; and chronic disease that should not be associated with air pollution may cause people to report excessive air pollution effects despite no differences in exposure compared to healthy people [
42]. Furthermore, Clougherty and colleagues [
43] reported higher rates of hypertension among women working in manufacturing jobs, and we found that hypertension was a strong predictor of cardinal symptom reporting among those occupationally exposed. Sex-related responses to workplace hazards and gender-related differences in work status are important considerations in this context. Therefore, and similar to the current study, Clougherty
et al. [
43] recognized the need to separate sex and gender effects on health when investigating the elevated health risks among women in manufacturing jobs as measured by onset of hypertension. They stratified the male and female sub-samples by propensity scores calculated from
a priori effects of gender on job status (hourly
versus salaried). The propensity scores were utilized to control for pre-hire gendered effects on job status, such as socioeconomic factors. They observed higher rates of hypertension only among women predicted to be hourly workers, which suggests that vulnerabilities are gendered in addition to potentially being sex-related. Unlike the current study they lacked information on chemical exposures, so it was not known whether sex-related responses to workplace hazards were due to differing exposures between men and women (which we try to show) or, for example, anatomical differences affecting safety equipment effectiveness.
The analytical approach utilized by Clougherty
et al. [
43] is a confirmatory, hypothesis-testing alternative to the technique utilized in the current study, which was to use CART to identify covariates that interacted with the binary construct male/female, and furthermore control for these covariates by stratification. To our knowledge no other study has utilized CART to investigate sex and gender effects on health outcomes related to air pollution specifically, but recursive partitioning methods have been used in other environmental health studies. For example, this methodology was used to identity subgroups with similar time-activity relationships to Volatile Organic Compound (VOC) exposure in the Exopolis study [
44]. Keegan
et al. [
45] used recursive partitioning to identify associations between the built environment and physical health, and Hu
et al. [
46] investigated the interactive effects of ambient temperature and sulphur dioxide on total mortality in Sydney, Australia by developing a time-series CART model. These studies demonstrate the utility of CART and similar techniques as an exploratory tool that can be used to identify variables of interest or interactions between specific predictor variables. The use of this technique is more widespread in clinical and diagnostic studies, but our application here suggests there may be opportunities for advancing its use in environmental health research. As applied to sex, gender and health research, recursive partitioning models offer a viable alternative to previously proposed analytical approaches, such as propensity analyses and multi-level modeling [
1], when lack of longitudinal data or study design does not permit using these techniques. Furthermore, CART can assist researchers to disentangle the effects of biology and culture in community-based studies where contextual factors may be influential.
Forastiere
et al. [
47] argues that short-term effects of air pollution on mortality are mainly due to pre-existing health conditions that increase susceptibility. The cross-sectional study design meant we were not able to investigate the etiology of chronic disease in our respondents, but other research has shown that prolonged exposure to air pollution can lead to asthma [
48]. One of the cardinal symptoms was wheezing or trouble breathing, so not surprisingly asthmatics were more likely to report 5+ CS.
Our results relating to different age groups and the relationship between sex, cardinal symptoms and air pollution in Sarnia should be interpreted with caution because of an inverse correlation between age and residential exposure, but a study conducted in Sweden found that the health-related quality of life was reduced by rhinitis and asthma more so in women than men aged 50–64 [
49]. Varying prevalence of pre-existing health conditions that increase susceptibility introduces another level of uncertainty to the modifying effects of occupational co-exposure on air pollution health outcomes. It is possible that socioeconomic or physiological determinants of chronic disease also influence selection of types of work that involve exposure to chemicals. In this study it was not possible to determine whether differences in susceptibility among occupationally exposed were due to sex, gender, or both, due to the cross-sectional study design. Nevertheless, the overall results support calls [
1,
50] for sex, gender and health considerations in environmental exposure research.