Inhibitory Activity of Marine Sponge-Derived Natural Products against Parasitic Protozoa
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
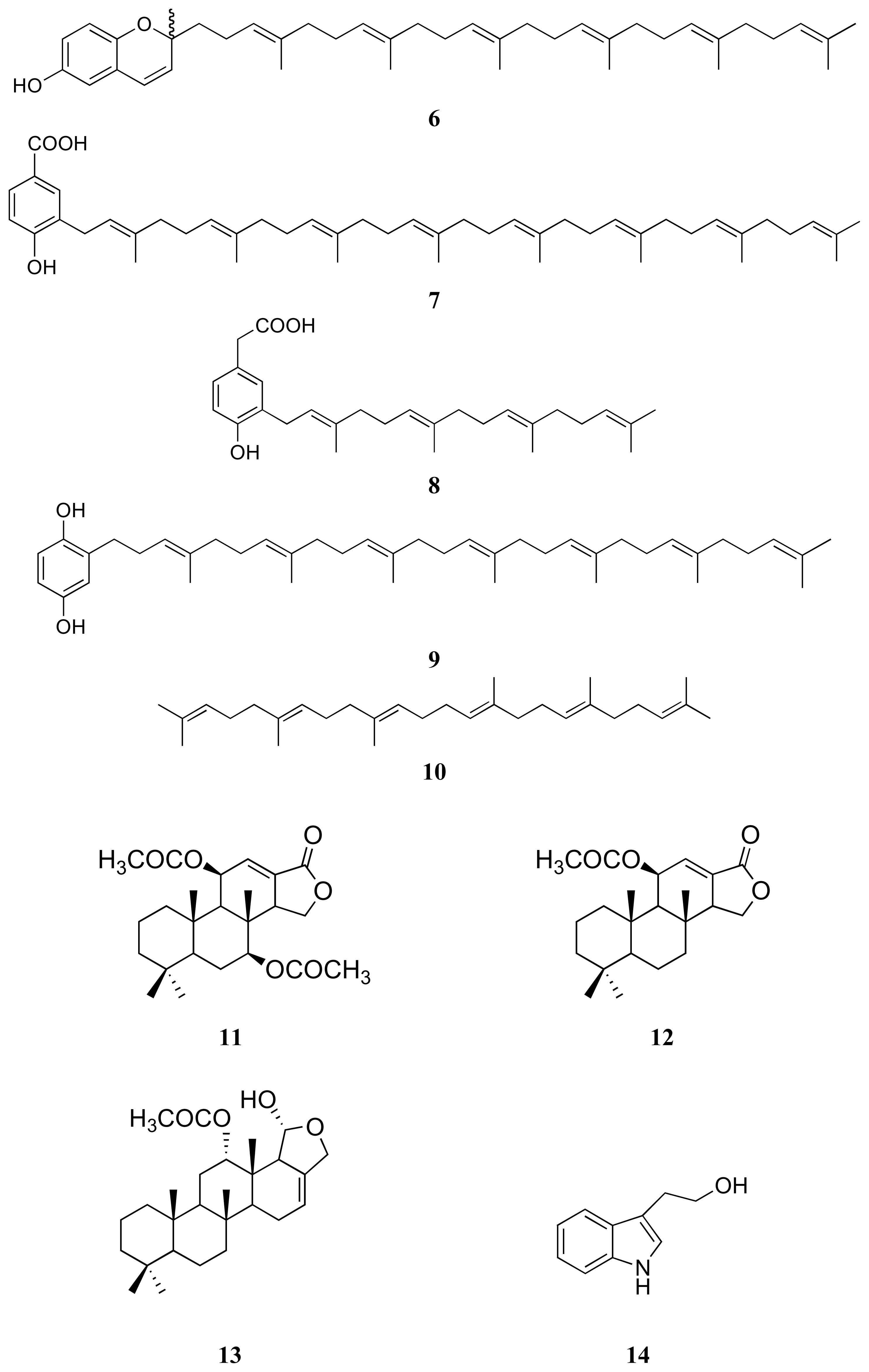
3.1. Isolation of compounds 1–14
3.2. Activity against Plasmodium falciparum
3.3. Activity against Trypanosoma brucei rhodesiense
3.4. Activity against Trypanosoma cruzi
3.5. Activity against Leishmania donovani
3.6. Cytotoxicity against L6 cells
4. Conclusions
- Samples Availability: Available from the authors.
References and Notes
- WHO. World malaria report; World Health Organization, 2008. Available online http://malaria.who.int/wmr2008/malaria2008.pdf (Accessed on 20 November 2009).
- D’Alessandro, U. Existing antimalarial agents and malaria-treatment strategies. Exp Opin Pharmacother 2009, 10, 1291–1306. [Google Scholar]
- Wongsrichanalai, C; Meshnick, SR. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg Infect Dis 2008, 14, 716–719. [Google Scholar]
- Stuart, K; Brun, R; Croft, S; Fairlamb, A; Gürtler, RE; McKerrow, J; Reed, S; Tarleton, R. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest 2008, 118, 1301–1310. [Google Scholar]
- Brun, R; Blum, J; Chappuis, F; Burri, C. Human African trypanosomiasis. Lancet 2009, in press. [Google Scholar]
- Bañuls, AL; Hide, M; Prugnolle, F. Leishmania and the leishmaniasis: A parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans. Adv Parasitol 2007, 64, 1–109. [Google Scholar]
- Mayer, AMS; Hamann, MT. Marine pharmacology in 2001–2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C: Pharmacol Toxicol 2002, 140, 265–286. [Google Scholar]
- Donia, M; Hamann, MT. Marine natural products and their potential applications as anti-infective agents. Lancet-Infec Dis 2003, 3, 338–348. [Google Scholar]
- Mayer, AM; Rodriguez, AD; Berlinck, RG; Hamann, MT. Marine pharmacology in 2003–4: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C: Pharmacol Toxicol 2007, 145, 553–581. [Google Scholar]
- Mayer, AM; Rodriguez, AD; Berlinck, RG; Hamann, MT. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Biochim Biophys Acta 2009, 1790, 283–308. [Google Scholar]
- Tasdemir, D; Topaloglu, B; Perozzo, R; Brun, R; O’Neill, R; Carballeira, NM; Zhang, X; Tonge, PJ; Linden, A; Rüedi, P. Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductases from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg Med Chem 2007, 15, 6834–6845. [Google Scholar]
- Del Rayo Camacho-Corona, M; Croft, SL; Phillipson, JD. Natural products as sources of antiprotozoal drugs. Curr Op Anti-inf Invest Drugs 2000, 2, 47–62. [Google Scholar]
- Salem, MM; Werbovetz, KA. Natural products from plants as drug candidates and lead compounds against leishmaniasis and trypanosomiasis. Curr Med Chem 2006, 13, 2571–2598. [Google Scholar]
- De Rosa, S; Mitova, M. Rahman, A-ur, Ed.; Bioactive marine sesterterpenoids. In Studies in Natural Products Chemistry; Elsevier Science Publishers: Amsterdam, Netherlands, 2005; Volume 32, pp. 109–168. [Google Scholar]
- Gochfeld, DJ; Hamann, MT. Isolation and biological evaluation of filiformin, plakortide F, and plakortone G from the Caribbean sponge Plakortis sp. J Nat Prod 2001, 64, 1477–1479. [Google Scholar]
- Benoit-Vical, F; Salery, M; Soh, PN; Ahond, A; Poupat, C. Girolline: A potential lead structure for antiplasmodial drug research. Planta Med 2008, 74, 438–444. [Google Scholar]
- Desoubzdanne, D; Marcourt, L; Raux, R; Chevalley, S; Dorin, D; Doerig, C; Valentin, A; Ausseil, F; Debitus, C. Alisiaquinones and alisiaquinol, dual inhibitors of Plasmodium falciparum enzyme targets from a new Caledonian deep water sponge. J Nat Prod 2008, 71, 1189–1192. [Google Scholar]
- Casapullo, A; Minale, L; Zollo, F. Paniceins and related sesquiterpenoids from the Mediterranean sponge Reniera fulva. J Nat Prod 1993, 56, 527–533. [Google Scholar]
- Urios, A; Largeron, M; Fleury, MB; Blanco, M. A convenient approach for evaluating the toxicity profiles of in vitro neuroprotective alkylaminophenol derivatives. Free Rad Biol Med 2006, 40, 791–800. [Google Scholar]
- Gonzalez, MA. Spongian diterpenes. Curr Bioact Compds 2007, 3, 1–36. [Google Scholar]
- Schmitz, FJ; Chang, JS; Hossain, MB; Van der Helm, D. Marine natural products: Spongiane derivatives from the sponge Igernella notabilis. J Org Chem 1985, 50, 2862–2865. [Google Scholar]
- Vik, A; Proszenyák, A; Vermeersch, M; Cos, P; Maes, L; Gundersen, LL. Screening of agelasine D and analogs for inhibitory activity against pathogenic protozoa; identification of hits for visceral leishmaniasis and Chagas disease. Molecules 2009, 14, 279–288. [Google Scholar]
- Akendengue, B; Ngou-Milama, E; Laurens, A; Hocquemiller, R. Recent advances in the fight against leishmaniasis with natural products. Parasites 1999, 6, 3–8. [Google Scholar]
- Staerk, D; Lemmich, E; Christensen, J; Kharazmi, A; Olsen, CE; Jaroszewski, JW. Leishmanicidal, antiplasmodial and cytotoxic activity of indole alkaloids from Corynanthe pachyceras. Planta Med 2000, 66, 531–536. [Google Scholar]
- Fournet, A; Munoz, V. Natural products as trypanocidal, antileishmanial and antimalarial drugs. Curr Top Med Chem 2002, 2, 1215–1237. [Google Scholar]
- Mishra, BB; Singh, RK; Srivastava, A; Tripathi, VS; Tiwari, VK. Fighting against leishmaniasis: Search of alkaloids as future true potential anti-leishmanial agents. Mini Rev Med Chem 2009, 9, 107–123. [Google Scholar]
- Erdogan, I; Şener, B; Higa, T. Tryptophol, a plant auxin isolated from the marine sponge Ircinia spinulosa. Biochem System Ecol 2000, 28, 793–794. [Google Scholar]
- Erdogan, I; Tanaka, J; Higa, T; Şener, B. Terpenoids from two sponge species of the Aegean Sea. Nat Prod Sci 1999, 5, 177–180. [Google Scholar]
- Matile, H; Pink, JRL. Lefkovits, I, Pernis, B, Eds.; Plasmodium falciparum malaria parasite cultures and their use in immunology. In Immunological Methods; Academic Press: San Diego, CA, USA, 1990; pp. 221–234. [Google Scholar]
- Baltz, T; Baltz, D; Giroud, C; Crockett, J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J 1985, 4, 1273–1277. [Google Scholar]
- Thuita, JK; Karanja, SM; Wenzler, T; Mdachi, RE; Ngotho, JM; Kagira, JM; Tidwell, R; Brun, R. Efficacy of the diamidine DB75 and its prodrug DB289, against murine models of human African trypanosomiasis. Acta Trop 2008, 108, 6–10. [Google Scholar]
- Räz, B; Iten, M; Grether-Bühler, Y; Kaminsky, R; Brun, R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense). Acta Trop 1997, 68, 139–147. [Google Scholar]
- Buckner, FS; Verlinde, CL; La Flamme, AC; van Voorhis, WC. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob Agents Chemother 1996, 40, 2592–2597. [Google Scholar]
- Mikus, J; Steverding, D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol Int 2000, 48, 265–269. [Google Scholar]

| Compound | Trypanosoma b. rhodesiense | Trypanosoma cruzi | Leishmania donovani | Plasmodium falciparum | Cytotoxicity |
|---|---|---|---|---|---|
| 1 | 27.45 | >90 | 14.2 | 11.18 | >90 |
| 2 | 16.16 | >90 | >90 | 3.51 | 34.0 |
| 3 | 13.94 | 17.49 | 4.80 | 14.02 | 27.45 |
| 4 | 17.94 | >90 | >90 | 7.51 | >90 |
| 5 | 4.90 | >90 | 10.2 | 13.36 | >90 |
| 6 | 41.43 | >90 | 15.9 | >20 | >90 |
| 7 | 13.11 | >90 | 5.60 | 1.57 | 86.48 |
| 8 | 0.60 | >90 | >90 | 3.30 | >90 |
| 9 | 3.54 | 4.08 | 18.9 | >20 | 2.62 |
| 10 | 15.03 | >90 | >90 | 1.16 | 34.89 |
| 11 | 2.47 | >90 | >90 | 0.43 | 3.93 |
| 12 | 4.14 | 4.51 | 0.75 | 1.09 | 3.32 |
| 13 | 55.25 | 40.43 | >90 | 7.48 | 60.33 |
| 14 | 5.89 | 49.37 | 9.60 | 5.08 | 63.46 |
| Standards | 0.003a | 0.359b | 0.20c | 0.056d | 0.004e |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Orhan, I.; Şener, B.; Kaiser, M.; Brun, R.; Tasdemir, D. Inhibitory Activity of Marine Sponge-Derived Natural Products against Parasitic Protozoa. Mar. Drugs 2010, 8, 47-58. https://doi.org/10.3390/md8010047
Orhan I, Şener B, Kaiser M, Brun R, Tasdemir D. Inhibitory Activity of Marine Sponge-Derived Natural Products against Parasitic Protozoa. Marine Drugs. 2010; 8(1):47-58. https://doi.org/10.3390/md8010047
Chicago/Turabian StyleOrhan, Ilkay, Bilge Şener, Marcel Kaiser, Reto Brun, and Deniz Tasdemir. 2010. "Inhibitory Activity of Marine Sponge-Derived Natural Products against Parasitic Protozoa" Marine Drugs 8, no. 1: 47-58. https://doi.org/10.3390/md8010047
APA StyleOrhan, I., Şener, B., Kaiser, M., Brun, R., & Tasdemir, D. (2010). Inhibitory Activity of Marine Sponge-Derived Natural Products against Parasitic Protozoa. Marine Drugs, 8(1), 47-58. https://doi.org/10.3390/md8010047





