Detailed NMR, Including 1,1-ADEQUATE, and Anticancer Studies of Compounds from the Echinoderm Colobometra perspinosa
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General experimental
3.2. Animal material
3.3. Bioassay
3.4. Extraction and isolation
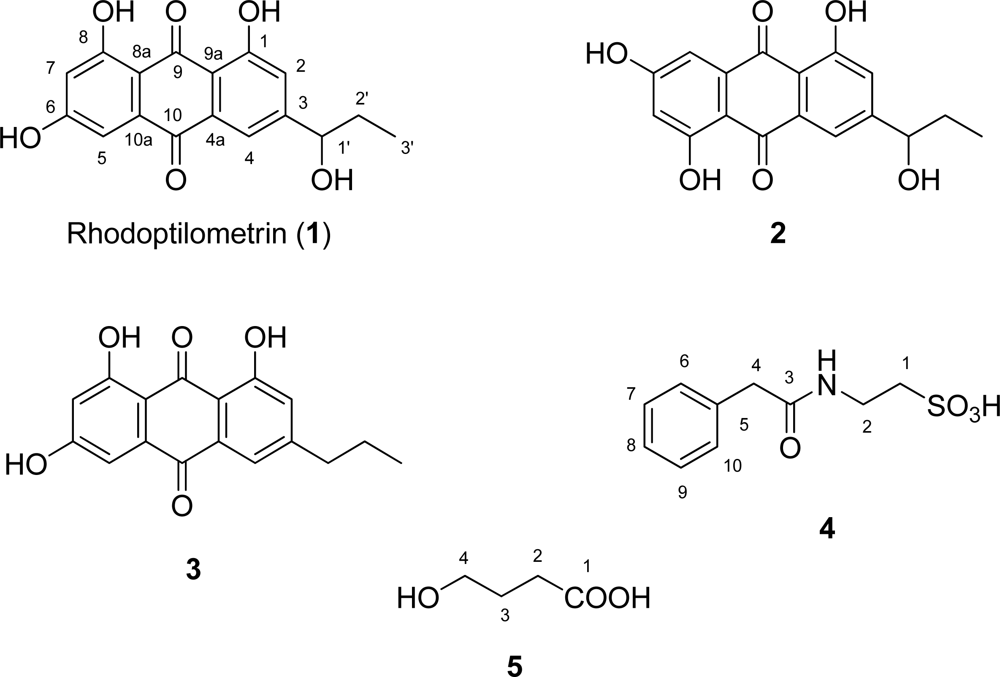
3.5. 3-(1’-Hydroxypropyl)-1,6,8-trihydroxy-9,10-anthraquinone (Rhodoptilometrin, 1)
3.6. 3-Propyl-1,6,8-trihydroxy-9,10-anthraquinone (3)
3.7. 4-[(Phenylacetyl)amino]ethanesulfonic acid (4)
3.8. 4-Hydroxybutanoic acid (5)
Acknowledgments
- Samples Availability: Available from the authors.
References and Notes
- Hay, ME; Duffy, JE; Pfister, AP; Fenical, W. Chemical defense against different marine herbivores: Are amphipods insect equivalents. Ecology 1986, 68, 1567–1580. [Google Scholar]
- de Nys, R; Steinberg, PD; Willemsen, P; Dworjanyn, SA; Gabelish, CL; King, RJ. Broad spectrum effects of secondary metabolites from the red alga Delisea pulchra in antifouling assays. Biofouling 1995, 8, 259–271. [Google Scholar]
- de Nys, R; Steinberg, PD. Recent advances in marine biotechnology. Biofilms, bioadhesion, corrosion and biofouling. In Role of Secondary Metabolites from Algae and Seagrasses in Biofouling Control; Fingerman, M, Nagabhushanam, R, Thompson, M-F, Eds.; Science Publishers: Enfield, UK, 1999; Volume 3, pp. 223–244. [Google Scholar]
- Pennings, SC; Pablo, SR; Paul, VJ. Chemical defenses of the tropical, benthic marine cyanobacterium Hormothamnion enteromorphoides: Diverse consumers and synergisms. Limnol Oceanogr 1997, 42, 911–917. [Google Scholar]
- Wright, AD; Wang, H; Gurrath, M; König, GM; Kocak, G; Neumann, G; Loria, P; Foley, M; Tilley, L. Inhibition of heme (FP) detoxification processes underlies the antimalarial activity of terpene isonitrile compounds from marine sponges. J Med Chem 2001, 44, 873–885. [Google Scholar]
- König, GM; Wright, AD; Linden, A. Antiplasmodial and cytotoxic metabolites from the maltese sponge. Agelas oroides Planta Medica 1998, 64, 443–447. [Google Scholar]
- Rinehart, KL; Morales, JJ; Reid, J; Reymundo, I; Floriano, P; Gravalos, LG. ETM-775 metabolite of ecteinascidin 743. United States Pat 6316214 2001. [Google Scholar]
- Powell, VH; Sutherland, MD. Pigments of Marine Animals. VI.* Anthraquinone pigments of the crinoids Ptilometra australis Wilton and Tropiometra afra Hartlaub. Aust J Chem 1967, 20, 541–543. [Google Scholar]
- Bartolini, GL; Erdman, TR; Scheuer, PJ. Anthraquinone pigments from the crinoid. Comanthus bennetti Tetrahedron 1973, 29, 3699–3702. [Google Scholar]
- Lee, NK; Kim, YH. New cytotoxic anthraquinones from the crinoid Ptilometra: 1’-Deoxyrhodoptilometrin-6-O-sulfate and rhodoptilometrin-6-O-sulfate. Bull Korean Chem Soc 1995, 16, 1011–1013. [Google Scholar]
- Köck, M; Reif, B; Gerlach, M; Reggelin, M. Application of the 1, n-ADEQUATE experiment in the assignment of highly substituted aromatic compounds. Molecules 1996, 1, 41–45. [Google Scholar]
- Cravedi, JP; Tulliez, J. Urinary metabolites of dodecylcyclohexane in Salmo gairdneri: Evidence of aromatization and taurine conjugation in trout. Xenobiotica 1987, 17, 1103–1111. [Google Scholar]
- Idle, JR; Millburn, P; Williams, R. Taurine conjugates as metabolites of arylacetic acids in the ferret. Xenobiotica 1978, 8, 253–264. [Google Scholar]
- Bessman, SP; Fishbein, WN. Gamma hydroxybutyrate, a normal brain metabolite. Nature 1963, 200, 1207–1208. [Google Scholar]
- Weil, A; Winifred, R. Depressants. In From Chocolate to Morphine, 2nd ed; Houghton Mifflin Company: Boston and New York, USA, 1993; p. 77. [Google Scholar]
- Benzer, TI. eMedicine: Toxicity, Gamma-Hydroxybutyrate. 2007.
- Mamelak, M; Scharf, M; Woods, M. Treatment of narcolepsy with gamma-hydroxybutyrate. A review of clinical and sleep laboratory findings. Sleep 1986, 9, 285–289. [Google Scholar]
- .
- Tapiolas, DM; Bowden, BF; Abou-Mansour, E; Willis, RH; Doyle, JR; Muirhead, AN; Liptrot, C; Llewellyn, LE; Wolff, CWW; Wright, AD; Motti, CA. Eusynstyelamides A, B and C, nNOS Inhibitors from the Ascidian Eusynstyela latericus. J Nat Prod 2009, 72, 1115–1120. [Google Scholar]
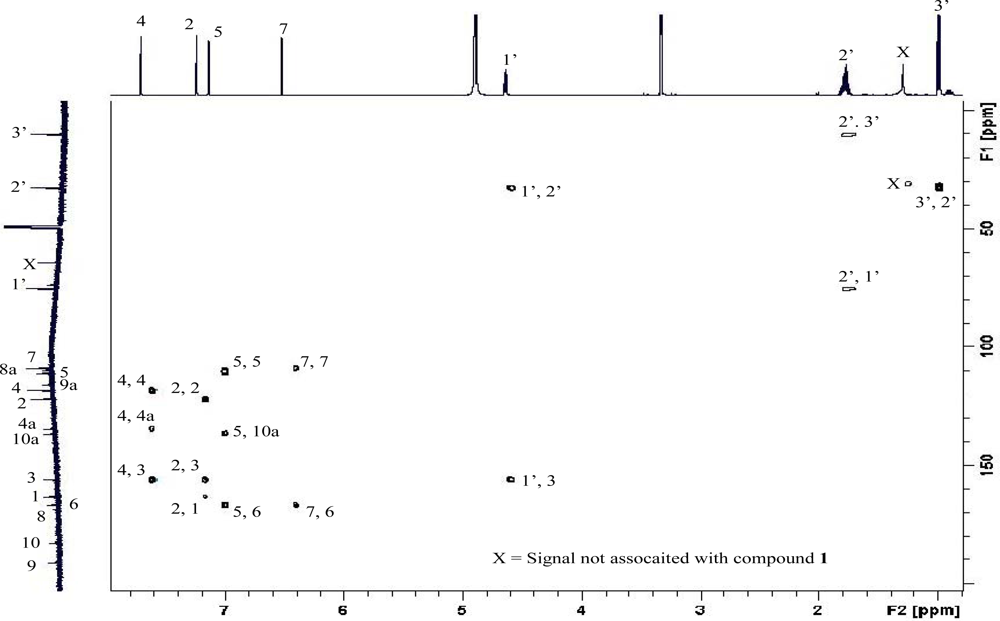
| 13C δ (m) | 1H δ (m, J Hz) | HMBC (6 Hz) | gHMBC (6 Hz) | 1,1-ADEQUATE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | 1a,10 | 1a | 3a | 1a,10 | 1a | 1b,9 | 1b | 1c,8 | 1a,10 | 1a | 1a |
| 1 | 163.7 (s) | 163.6 (s) | 163.6 (s) | ||||||||
| 2 | 122.4 (d) | 122.3 (d) | 124.7 (d) | 7.20 (s) | 7.24 (d, 1.4) | 7.17 (brs) | 7.24 (d, 1.4) | 6.73 (d, 1.2) | C-4, 1’ | C-1’, 1, 3, 4, 9a | C-1, 3 |
| 3 | 156.1 (s) | 156.6 (s) | 154.2 (s) | ||||||||
| 4d | 116.6 (d) | 118.4 (d) | 121.2 (d) | 7.70 (s) | 7.72 (d, 1.4) | 7.56 (brs) | 7.66 (d, 1.4) | 7.16 (d, 1.2) | C-2, 10, 1’ | C-1’, 1, 2, 9, 9a, 10 | C-3, 4a |
| 4a | 135.3 (s) | 134.9 (s) | 134.7 (s) | ||||||||
| 5d | 113.6 (d) | 110.4 (d) | 109.1 (d) | 7.09 (s) | 7.16 (d, 2.4) | 6.96 (d, 2.0) | 7.02 (d, 2.5) | 6.61 (d 2.0) | C-10 | C-6, 7, 8, 8a, 9, 10 | C-6, 10a |
| 6d | 160.2 (s) | 166.7 (s) | 166.6 (s) | ||||||||
| 7 | 109.7 (d) | 109.1 (d) | 110.1 (d) | 6.35 (d, 3.4) | 6.52 (d, 2.4) | 6.41 (d 2.0) | 6.44 (d, 2.5) | 6.02 (d, 2.0) | C-5 | C-5, 6, 8, 8a | C-6 |
| 8 | 167.5 (s) | 167.9 (s) | 167.2 (s) | ||||||||
| 8ad | 113.9 (s) | 110.5 (s) | 110.5 (s) | ||||||||
| 9 | 192.0 (s) | 191.7 (s) | 191.9 (s) | ||||||||
| 9ad | 118.4 (s) | 115.9 (s) | 115.0 (s) | ||||||||
| 10 | 184.3 (s) | 183.2 (s) | 183.2 (s) | ||||||||
| 10a | 136.9 (s) | 136.9 (s) | 136.9 (s) | ||||||||
| 1′ | 75.5 (d) | 75.4 (d) | 39.2 (t) | 4.60 (m) | 4.62 (t, 6.4) | 4.56 (m) | 4.57 (t, 6.5) | 4.19 (t, 5.8) | C-2, 4 | C-2’, 3’, 2, 3, 4 | C-2’, 3 |
| 2′ | 32.6 (t) | 32.6 (t) | 24.8 (t) | 1.73 (m) | 1.75 (m) | 1.65 (m) | 1.63 (m) | C-3, 3′ | C-1’, 3’, 3 | C-1’, 3’ | |
| 3′ | 10.3 (q) | 10.2 (q) | 14.0 (q) | 0.94 (t, 12.5) | 0.96 (t, 7.6) | 0.91 (t, 8.0) | 0.86 (t, 7.3) | C-1’, 2′ | C-1’, 2’ | C-2’ | |
| 13C δ (m) for 1 in CD3OD | ||||||
|---|---|---|---|---|---|---|
| No. | Unknowna | Unknown | conc mg/600 μL | 8 | 4 | |
| 32 | 16 | |||||
| 1 | 163.7 (s) | 163.6 (s) | 163.4 (s) | 163.5 (s) | 163.6 (s) | 163.7 (s) |
| 2 | 122.4 (d) | 122.3 (d) | 122.2 (d) | 122.2 (d) | 122.3 (d) | 122.3 (d) |
| 3 | 156.1 (s) | 156.6 (s) | 156.5 (s) | 156.6 (s) | 156.6 (s) | 156.7 (s) |
| 4b | 116.6 (d) | 118.4 (d) | 118.4 (d) | 118.4 (d) | 118.4 (d) | 118.4 (d) |
| 4a | 135.3 (s) | 134.9 (s) | 134.5 (s) | 134.7 (s) | 134.8 (s) | 134.8 (s) |
| 5b | 113.6 (d) | 110.4 (d) | 110.4 (d) | 110.4 (d) | 110.4 (d) | 110.4 (d) |
| 6b | 160.2 (s) | 166.7 (s) | 166.4 (s) | 166.5 (s) | 166.6 (s) | 166.7 (s) |
| 7 | 109.7 (d) | 109.1 (d) | 109.0 (d) | 109.0 (d) | 109.0 (d) | 109.1 (d) |
| 8 | 167.5 (s) | 167.9 (s) | 167.1 (s) | 167.2 (s) | 167.3 (s) | 167.4 (s) |
| 8ab | 113.9 (s) | 110.5 (s) | 110.3 (s) | 110.4 (s) | 110.5 (s) | 110.6 (s) |
| 9 | 192.0 (s) | 191.7 (s) | 191.5 (s) | 191.7 (s) | 191.8 (s) | 191.9 (s) |
| 9ab | 118.4 (s) | 115.9 (s) | 115.6 (s) | 115.7 (s) | 115.8 (s) | 115.9 (s) |
| 10 | 184.3 (s) | 183.2 (s) | 182.7 (s) | 182.9 (s) | 183.0 (s) | 183.1 (s) |
| 10a | 136.9 (s) | 136.9 (s) | 136.6 (s) | 136.7 (s) | 136.9 (s) | 137.0 (s) |
| 1′ | 75.5 (d) | 75.4 (d) | 75.4 (d) | 75.4 (d) | 75.4 (d) | 75.4 (d) |
| 2′ | 32.6 (t) | 32.6 (t) | 32.6 (t) | 32.6 (t) | 32.6 (t) | 32.6 (t) |
| 3′ | 10.3 (q) | 10.2 (q) | 10.2 (q) | 10.2 (q) | 10.2 (q) | 10.2 (q) |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wright, A.D.; Nielson, J.L.; Tapiolas, D.M.; Motti, C.A.; Ovenden, S.P.B.; Kearns, P.S.; Liptrot, C.H. Detailed NMR, Including 1,1-ADEQUATE, and Anticancer Studies of Compounds from the Echinoderm Colobometra perspinosa. Mar. Drugs 2009, 7, 565-575. https://doi.org/10.3390/md7040565
Wright AD, Nielson JL, Tapiolas DM, Motti CA, Ovenden SPB, Kearns PS, Liptrot CH. Detailed NMR, Including 1,1-ADEQUATE, and Anticancer Studies of Compounds from the Echinoderm Colobometra perspinosa. Marine Drugs. 2009; 7(4):565-575. https://doi.org/10.3390/md7040565
Chicago/Turabian StyleWright, Anthony D., Jonathan L. Nielson, Dianne M. Tapiolas, Cherie A. Motti, Simon P. B. Ovenden, Philip S. Kearns, and Catherine H. Liptrot. 2009. "Detailed NMR, Including 1,1-ADEQUATE, and Anticancer Studies of Compounds from the Echinoderm Colobometra perspinosa" Marine Drugs 7, no. 4: 565-575. https://doi.org/10.3390/md7040565




