A Sesquiterpene Quinone, 5-Epi-smenospongine, Promotes TNF-α Production in LPS-stimulated RAW 264.7 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Compounds
2.2. Cell lines and culture conditions
2.3. Detection of murine TNF-α by ELISA
2.4. Determination of cell proliferation
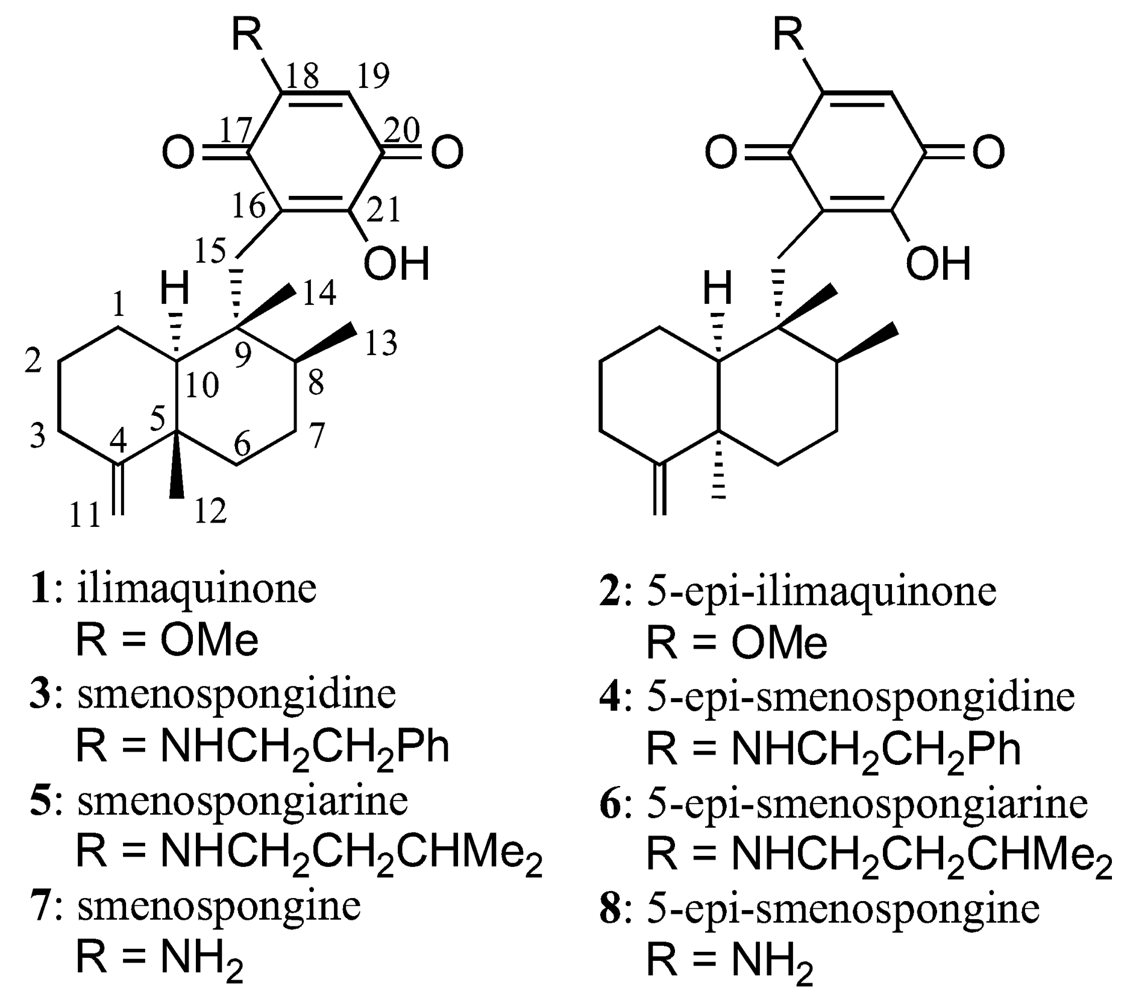
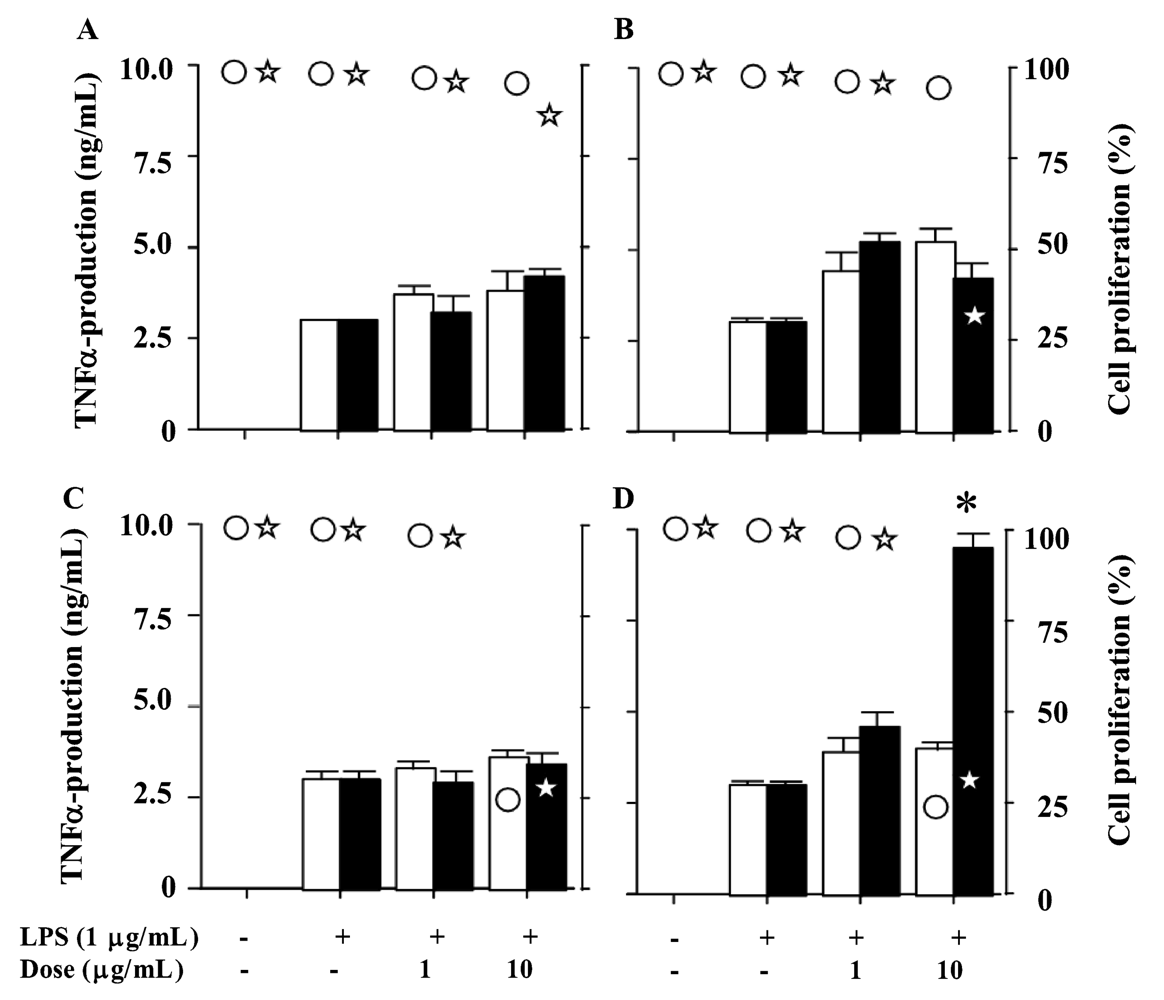
3. Results and Discussion
Acknowledgements
- Samples Availability: Not available.
References
- Luibrand, RT; Erdman, TR; Vollmer, JJ; Scheuer, PJ; Finer, J; Clardy, J. Ilimaquinone, a sesquiterpenoid quinone from a marine sponge. Tetrahedron 1979, 35, 609–612. [Google Scholar]
- Capon, RJ; MacLeod, JK. A revision of the absolute stereochemistry of ilimaquinone. J Org Chem 1987, 52, 5059–5060. [Google Scholar]
- Carté, B; Rose, CB; Faulkner, DJ. 5-epi-Ilimaquinone, a metabolite of the sponge Fenestraspongia Sp. J Org Chem 1985, 50, 2785–2787. [Google Scholar]
- Rodríguez, J; Quiñoá, E; Riguera, R; Peters, BM; Abrell, LM; Crews, P. The structures and stereochemistry of cytotoxic sesquiterpene quinones from Dactylospongia elegans. Tetrahedron 1992, 48, 6667–6680. [Google Scholar]
- Kondracki, M-L; Guyot, M. Biologically active quinone and hydroquinone sesquiterpenoids from the sponge Smenospongia sp. Tetrahedron 1989, 45, 1995–2004. [Google Scholar]
- Kondracki, M-L; Guyot, M. Smenospongine: a cytotoxic and antimicrobial aminoquinone isolated from Smenospongia sp. Tetrahedron Lett 1987, 28, 5815–5818. [Google Scholar]
- Mitome, H; Nagasawa, T; Miyaoka, H; Yamada, Y; van Soest, RW. Dactyloquinones A and B, new sesquiterpenoid quinones from the Okinawan marine sponge Dactylospongia elegans. J Nat Prod 2001, 64, 1506–1508. [Google Scholar]
- Salmoun, M; Devijver, C; Daloze, D; Braekman, JC; Gomez, R; de Kluijver, M; van Soest, RWM. New sesquiterpene/quinones from two sponges of the genus Hyrtios. J Nat Prod 2000, 63, 452–456. [Google Scholar]
- Kwak, JH; Schmitz, FJ; Kelly, M. Sesquiterpene quinols/quinones from the Micronesian sponge Petrosaspongia metachromia. J Nat Prod 2000, 63, 1153–1156. [Google Scholar]
- Oda, T; Wang, W; Fujita, A; Mochizuki, M; Ukai, K; Namikoshi, M. Promotion of IL-8 production in PMA-stimulated HL-60 cells by sesquiterpene quinones from a marine sponge Hippospongia sp. J Nat Med 2007, 61, 434–437. [Google Scholar]
- Graves, DT; Jiang, Y. Chemokines, a family of chemotactic cytokines. Crit Rev Oral Biol Med 1995, 6, 109–118. [Google Scholar]
- di Celle, PF; Carbone, A; Marchis, D; Zhou, D; Sozzani, S; Zupo, S; Pini, M; Mantovani, A; Foa, R. Cytokine gene expression in B-cell chronic lymphocytic leukemia: evidence of constitutive interleukin-8 (IL-8) mRNA expression and secretion of biologically active IL-8 protein. Blood 1994, 84, 220–228. [Google Scholar]
- Green, AR; Green, VL; White, MC; Speirs, V. Expression of Cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer 1997, 72, 937–941. [Google Scholar]
- Konig, B; Steinbach, F; Janocha, B; Drynda, A; Stumm, M; Philipp, C; Allhoff, EP; Konig, W. The differential expression of proinflammatory cytokines IL-6, IL-8 and TNF-alpha in renal cell carcinoma. Anticancer Res 1999, 19, 1519–1524. [Google Scholar]
- Galffy, G; Mohammed, KA; Dowling, PA; Nasreen, N; Ward, MJ; Antony, VB. Interleukin 8: an autocrine growth factor for malignant mesothelioma. Cancer Res 1999, 59, 367–371. [Google Scholar]
- Carmichael, J; DeGraff, WG; Gazdar, AF; Minna, JD; Mitchell, JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 1987, 47, 936–942. [Google Scholar]
- Prokofeva, NG; Utkina, NK; Chaikina, EL; Makarchenko, AE. Biological activities of marine sesquiterpenoid quinones: structure-activity relationships in cytotoxic and hemolytic assays. Comp Biochem Physiol Bio Mol Biol 2004, 139, 169–173. [Google Scholar]
- Tziveleka, LA; Vagias, C; Roussis, V. Natural products with anti-HIV activity from marine organisms. Curr Top Med Chem 2003, 3, 1512–1535. [Google Scholar]
- Pous, C; Chabin, K; Drechou, A; Barbot, L; Phung-Koskas, T; Settegrana, C; Bourguet-Kondracki, ML; Maurice, M; Cassio, D; Guyot, M; Durand, G. Functional specialization of stable and dynamic microtubules in protein traffic in WIF-B cells. J Cell Biol 1998, 142, 153–165. [Google Scholar]
- Cruciani, V; Mikalsen, SO. Ilimaquinone inhibits gap junctional communication in a connexin isotype-specific manner. Exp Cell Res 2005, 304, 136–148. [Google Scholar]
- Nambiar, MP; Wu, HC. Ilimaquinone inhibits the cytotoxicities of ricin, diphtheria toxin, and other protein toxins in Vero cells. Exp Cell Res 1995, 219, 671–678. [Google Scholar]
- Aoki, S; Kong, D; Matsui, K; Rachmat, R; Kobayashi, M. Sesquiterpene aminoquinones, from a marine sponge, induce erythroid differentiation in human chronic myelogenous leukemia, K562 cells. Chem Pharm Bull 2004, 52, 935–937. [Google Scholar]
Share and Cite
Oda, T.; Wang, W.; Ukai, K.; Nakazawa, T.; Mochizuki, M. A Sesquiterpene Quinone, 5-Epi-smenospongine, Promotes TNF-α Production in LPS-stimulated RAW 264.7 Cells. Mar. Drugs 2007, 5, 151-156. https://doi.org/10.3390/md504151
Oda T, Wang W, Ukai K, Nakazawa T, Mochizuki M. A Sesquiterpene Quinone, 5-Epi-smenospongine, Promotes TNF-α Production in LPS-stimulated RAW 264.7 Cells. Marine Drugs. 2007; 5(4):151-156. https://doi.org/10.3390/md504151
Chicago/Turabian StyleOda, Taiko, Weifang Wang, Kazuyo Ukai, Takahiro Nakazawa, and Masataka Mochizuki. 2007. "A Sesquiterpene Quinone, 5-Epi-smenospongine, Promotes TNF-α Production in LPS-stimulated RAW 264.7 Cells" Marine Drugs 5, no. 4: 151-156. https://doi.org/10.3390/md504151



