Marine Brown Algae-Derived Compounds as Potential Inhibitors of Japanese Encephalitis Virus RNA-Dependent RNA Polymerase
Abstract
:1. Introduction
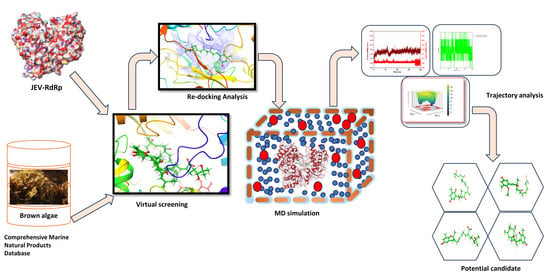
2. Results
2.1. Virtual Screening Analysis
2.2. Re-Docking and Intermolecular Interaction Studies
2.3. Molecular Dynamic Simulation Studies
2.3.1. RMSD and RMSF Analysis
2.3.2. Hydrogen Bond Analysis
2.4. MM/PBSA Calculation
2.5. Principal Component Analysis
2.6. Free Energy Landscape
3. Discussion
4. Materials and Methods
4.1. Virtual Screening
4.2. Re-Docking
4.3. Molecular Dynamics Simulation
4.4. MMPBSA Calculation
4.5. Principal Component Analysis (PCA) and Free Energy Landscape (FEL)
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Japanese Encephalitis. Available online: https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis (accessed on 6 February 2024).
- Campbell, G.; Hills, S.; Fischer, M.; Jacobson, J.; Hoke, C.; Hombach, J.; Marfin, A.; Solomon, T.; Tsai, T.; Tsui, V.; et al. Estimated Global Incidence of Japanese Encephalitis. Bull. World Health Org. 2011, 89, 766–774. [Google Scholar] [CrossRef]
- FAQ—JAPANESE ENCEPHALITIS :: National Center for Vector Borne Diseases Control (NCVBDC). Available online: https://ncvbdc.mohfw.gov.in/index1.php?lang=1&level=2&sublinkid=5930&lid=4005 (accessed on 6 February 2024).
- Ghosh, D.; Basu, A. Japanese Encephalitis—A Pathological and Clinical Perspective. PLoS Negl. Trop. Dis. 2009, 3, e437. [Google Scholar] [CrossRef]
- Surana, P.; Satchidanandam, V.; Nair, D.T. RNA-Dependent RNA Polymerase of Japanese Encephalitis Virus Binds the Initiator Nucleotide GTP to Form a Mechanistically Important Pre-Initiation State. Nucleic Acids Res. 2014, 42, 2758–2773. [Google Scholar] [CrossRef] [PubMed]
- Joe, S.; Salam, A.A.A.; Neogi, U.; Naren Babu, N.; Mudgal, P.P. Antiviral Drug Research for Japanese Encephalitis: An Updated Review. Pharmacol. Rep. 2022, 74, 273–296. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising Bioactive Compounds from the Marine Environment and Their Potential Effects on Various Diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Rodríguez, A.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2009–2011: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T. Impact of Marine Chemical Ecology Research on the Discovery and Development of New Pharmaceuticals. Mar. Drugs 2023, 21, 174. [Google Scholar] [CrossRef] [PubMed]
- Alawam, A.S.; Alawam, H.S.; Alshahrani, M.M.; Alwethaynani, M.S.; Alneghery, L.M.; Alamri, M.A. Structural and Dynamical Basis of VP35-RBD Inhibition by Marine Fungi Compounds to Combat Marburg Virus Infection. Mar. Drugs 2024, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Gattan, H.S.; Mahmoud Alawi, M.; Bajrai, L.H.; Alandijany, T.A.; Alsaady, I.M.; El-Daly, M.M.; Dwivedi, V.D.; Azhar, E.I. A Multifaceted Computational Approach to Understanding the MERS-CoV Main Protease and Brown Algae Compounds’ Interaction. Mar. Drugs 2023, 21, 626. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Guo, J.; Wang, P.; Zhang, L.; Xiao, G.; Wang, W. Screening of FDA-Approved Drugs for Inhibitors of Japanese Encephalitis Virus Infection. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Zandi, K.; Bassit, L.; Amblard, F.; Cox, B.D.; Hassandarvish, P.; Moghaddam, E.; Yueh, A.; Libanio Rodrigues, G.O.; Passos, I.; Costa, V.V. Nucleoside Analogs with Selective Antiviral Activity against Dengue Fever and Japanese Encephalitis Viruses. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Sadybekov, A.V.; Katritch, V. Computational Approaches Streamlining Drug Discovery. Nature 2023, 616, 673–685. [Google Scholar] [CrossRef]
- Gago, F. Computational Approaches to Enzyme Inhibition by Marine Natural Products in the Search for New Drugs. Mar. Drugs 2023, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Parate, S.; Yoon, S.; Lee, G.; Lee, K.W. Computational Simulations Identified Marine-Derived Natural Bioactive Compounds as Replication Inhibitors of SARS-CoV-2. Front. Microbiol. 2021, 12, 647295. [Google Scholar] [CrossRef]
- Remya, R.R.; Samrot, A.V.; Kumar, S.S.; Mohanavel, V.; Karthick, A.; Chinnaiyan, V.K.; Umapathy, D.; Muhibbullah, M. Bioactive Potential of Brown Algae. Adsorpt. Sci. Technol. 2022, 2022, 9104835. [Google Scholar] [CrossRef]
- Hakim, M.M.; Patel, I.C. A Review on Phytoconstituents of Marine Brown Algae. Futur. J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- Catarino, M.D.; Marçal, C.; Bonifácio-Lopes, T.; Campos, D.; Mateus, N.; Silva, A.M.S.; Pintado, M.M.; Cardoso, S.M. Impact of Phlorotannin Extracts from Fucus Vesiculosus on Human Gut Microbiota. Mar. Drugs 2021, 19, 375. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine Natural Products. Nat. Prod. Rep. 1988, 5, 613–663. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, S.; Lurong, Q.; Qi, Z. Recent Advances in Antivirals for Japanese Encephalitis Virus. Viruses 2023, 15, 1033. [Google Scholar] [CrossRef]
- Binderup, A.; Galli, A.; Fossat, N.; Fernandez-Antunez, C.; Mikkelsen, L.S.; Rivera-Rangel, L.R.; Scheel, T.K.; Fahnøe, U.; Bukh, J.; Ramirez, S. Differential Activity of Nucleotide Analogs against Tick-Borne Encephalitis and Yellow Fever Viruses in Human Cell Lines. Virology 2023, 585, 179–185. [Google Scholar] [CrossRef]
- Julander, J.G.; Demarest, J.F.; Taylor, R.; Gowen, B.B.; Walling, D.M.; Mathis, A.; Babu, Y. An Update on the Progress of Galidesivir (BCX4430), a Broad-Spectrum Antiviral. Antivir. Res. 2021, 195, 105180. [Google Scholar] [CrossRef]
- Srivastava, K.S.; Jeswani, V.; Pal, N.; Bohra, B.; Vishwakarma, V.; Bapat, A.A.; Patnaik, Y.P.; Khanna, N.; Shukla, R. Japanese Encephalitis Virus: An Update on the Potential Antivirals and Vaccines. Vaccines 2023, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections. Chem.–Asian J. 2019, 14, 3962–3968. [Google Scholar] [CrossRef]
- Deshpande, S.; Huo, W.; Shrestha, R.; Sparrow, K.; Evans, G.B.; Harris, L.D.; Kingston, R.L.; Bulloch, E.M. Inhibition of the DENV2 and ZIKV RNA Polymerases by Galidesivir Triphosphate Measured Using a Continuous Fluorescence Assay. bioRxiv 2022, 2022.12.20.521302. [Google Scholar]
- Warren, T.K.; Wells, J.; Panchal, R.G.; Stuthman, K.S.; Garza, N.L.; Van Tongeren, S.A.; Dong, L.; Retterer, C.J.; Eaton, B.P.; Pegoraro, G. Protection against Filovirus Diseases by a Novel Broad-Spectrum Nucleoside Analogue BCX4430. Nature 2014, 508, 402–405. [Google Scholar] [CrossRef]
- Dwivedi, V.D.; Singh, A.; El-Kafraway, S.A.; Alandijany, T.A.; Faizo, A.A.; Bajrai, L.H.; Kamal, M.A.; Azhar, E.I. Mechanistic Insights into the Japanese Encephalitis Virus RNA Dependent RNA Polymerase Protein Inhibition by Bioflavonoids from Azadirachta Indica. Sci. Rep. 2021, 11, 18125. [Google Scholar] [CrossRef]
- Yadav, P.; El-Kafrawy, S.A.; El-Day, M.M.; Alghafari, W.T.; Faizo, A.A.; Jha, S.K.; Dwivedi, V.D.; Azhar, E.I. Discovery of Small Molecules from Echinacea Angustifolia Targeting RNA-Dependent RNA Polymerase of Japanese Encephalitis Virus. Life 2022, 12, 952. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Chen, Z. Insights into the Molecular Mechanisms of Protein-Ligand Interactions by Molecular Docking and Molecular Dynamics Simulation: A Case of Oligopeptide Binding Protein. Comput. Math. Methods Med. 2018, 2018, 3502514. [Google Scholar] [CrossRef]
- Schrödinger Release Maestro; Schrödinger, LLC: New York, NY, USA, 2021.
- Maisuradze, G.G.; Liwo, A.; Scheraga, H.A. Relation between Free Energy Landscapes of Proteins and Dynamics. J. Chem. Theory Comput. 2010, 6, 583–595. [Google Scholar] [CrossRef]
- Higo, J.; Ikebe, J.; Kamiya, N.; Nakamura, H. Enhanced and Effective Conformational Sampling of Protein Molecular Systems for Their Free Energy Landscapes. Biophys. Rev. 2012, 4, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, E.; Mereghetti, P.; Fantucci, P.; Grandori, R.; De Gioia, L. Free-Energy Landscape, Principal Component Analysis, and Structural Clustering to Identify Representative Conformations from Molecular Dynamics Simulations: The Myoglobin Case. J. Mol. Graph. Model. 2009, 27, 889–899. [Google Scholar] [CrossRef]
- Kumari, M.; Singh, R.; Subbarao, N. Exploring the Interaction Mechanism between Potential Inhibitor and Multi-Target Mur Enzymes of Mycobacterium Tuberculosis Using Molecular Docking, Molecular Dynamics Simulation, Principal Component Analysis, Free Energy Landscape, Dynamic Cross-Correlation Matrices, Vector Movements, and Binding Free Energy Calculation. J. Biomol. Struct. Dyn. 2022, 40, 13497–13526. [Google Scholar]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and Scoring in Virtual Screening for Drug Discovery: Methods and Applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, E.; Maroufi, P.; Khodadadi, E.; Esposito, I.; Ganbarov, K.; Espsoito, S.; Yousefi, M.; Zeinalzadeh, E.; Kafil, H.S. Study of Combining Virtual Screening and Antiviral Treatments of the SARS-CoV-2 (COVID-19). Microb. Pathog. 2020, 146, 104241. [Google Scholar] [CrossRef]
- Prasertsuk, K.; Prongfa, K.; Suttiwanich, P.; Harnkit, N.; Sangkhawasi, M.; Promta, P.; Chumnanpuen, P. Computer-Aided Screening for Potential Coronavirus 3-Chymotrypsin-like Protease (3CLpro) Inhibitory Peptides from Putative Hemp Seed Trypsinized Peptidome. Molecules 2023, 28, 50. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Metwaly, A.M.; Hassan, A.; Abd El-Aziz, T.M.; Elkaeed, E.B.; Eissa, I.H.; Arafa, R.K.; Stockand, J.D. Comprehensive Virtual Screening of the Antiviral Potentialities of Marine Polycyclic Guanidine Alkaloids against SARS-CoV-2 (COVID-19). Biomolecules 2021, 11, 460. [Google Scholar] [CrossRef]
- Harnkit, N.; Khongsonthi, T.; Masuwan, N.; Prasartkul, P.; Noikaew, T.; Chumnanpuen, P. Virtual Screening for SARS-CoV-2 Main Protease Inhibitory Peptides from the Putative Hydrolyzed Peptidome of Rice Bran. Antibiotics 2022, 11, 1318. [Google Scholar] [CrossRef]
- Veljkovic, N.; Glisic, S.; Perovic, V.; Veljkovic, V. The Role of Long-Range Intermolecular Interactions in Discovery of New Drugs. Expert Opin. Drug Discov. 2011, 6, 1263–1270. [Google Scholar] [CrossRef]
- Yunta, M. It Is Important to Compute Intramolecular Hydrogen Bonding in Drug Design? Am. J. Model. Optim. 2017, 5, 24–57. [Google Scholar] [CrossRef]
- Labbé, C.M.; Rey, J.; Lagorce, D.; Vavruša, M.; Becot, J.; Sperandio, O.; Villoutreix, B.O.; Tufféry, P.; Miteva, M.A. MTiOpenScreen: A Web Server for Structure-Based Virtual Screening. Nucleic Acids Res. 2015, 43, W448–W454. [Google Scholar] [CrossRef]
- Lyu, C.; Chen, T.; Qiang, B.; Liu, N.; Wang, H.; Zhang, L.; Liu, Z. CMNPD: A Comprehensive Marine Natural Products Database towards Facilitating Drug Discovery from the Ocean. Nucleic Acids Res. 2021, 49, D509–D515. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2. 0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 All-Atom Additive Protein Force Field: Validation Based on Comparison to NMR Data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]







| Compound ID | Compound Name | Compound Source Species Name |
|---|---|---|
| CMNPD16749 | Sargothunbergol A | Sargassum thunbergii [20] |
| CMNPD2606 | Amentadione | Carpodesmia amentacea [21] |
| CMNPD27817 | Cystone A | Cystoseira usneoides [22] |
| CMNPD23662 | Cystodione C | Cystoseira usneoides [23] |
| Serial Number | Complex | H-Bond | Hydrophobic | π–π Stacking/ *π–Cation Interaction | Salt Bridge | Glycine |
|---|---|---|---|---|---|---|
| 1. | JEV–RdRp–CMNPD16749 | Val607, Cys714, Arg742, Ser804 | Leu411, Ala413, Val514, Val607, Val608, Tyr610, Cys714, Leu739, Met766, Leu770, Tyr771, Trp800, Ile802 | -- | Lys463, Arg734, Arg742 | Gly412, Gly605 |
| 2. | JEV–RdRp–CMNPD2606 | Ala475, Arg460, Val607, Ile802 | Leu411, Ala413, Val414, Ala475, Ile476, Trp477, Val607, Val608, Tyr610, Cys714, Trp800, Ile802 | -- | -- | Gly412, Gly603, Gly605 |
| 3. | JEV–RdRp–CMNPD27817 | Ala413, Arg474, Val607, Tyr610, Arg742, Ser799, Ile802 | Ala410, Leu411, Ala413, Val607, Val608, Tyr610, Ala611, Cys714, Trp800, Ile802 | Lys463* | -- | Gly412 |
| 4. | JEV–RdRp–CMNPD23662 | Gln606, Trp800, Ile802 | Leu411, Ala413, Val607, Tyr610, Cys714, Trp800, Ile802 | -- | -- | Gly412, Gly605 |
| 5. | JEV–RdRp–galidesivir | Pro464, Arg734, Gly735,Glu738 | Ala470, Pro464, Pro712, Pro713, Cys714 | -- | -- | Gly465, Gly735 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, S.O. Marine Brown Algae-Derived Compounds as Potential Inhibitors of Japanese Encephalitis Virus RNA-Dependent RNA Polymerase. Mar. Drugs 2024, 22, 92. https://doi.org/10.3390/md22020092
Alshammari SO. Marine Brown Algae-Derived Compounds as Potential Inhibitors of Japanese Encephalitis Virus RNA-Dependent RNA Polymerase. Marine Drugs. 2024; 22(2):92. https://doi.org/10.3390/md22020092
Chicago/Turabian StyleAlshammari, Saud O. 2024. "Marine Brown Algae-Derived Compounds as Potential Inhibitors of Japanese Encephalitis Virus RNA-Dependent RNA Polymerase" Marine Drugs 22, no. 2: 92. https://doi.org/10.3390/md22020092