Structures and Anti-Allergic Activities of Natural Products from Marine Organisms
Abstract
:1. Introduction
2. Material and Methodology for Literature Survey
3. Chemical Structures and Biological Properties of the Anti-Allergy Compounds Linked with Marine Plants, Marine Microorganisms, and Marine Animals
3.1. Marine Plants
3.1.1. Natural Products Derived from Marine Plants with Anti-Allergic Activity
3.1.2. Crude Extracts from Marine Plants as Potential Sources with Anti-Allergic Activity
3.2. Marine Animals
3.2.1. Natural Products Derived from Marine Animals with Anti-Allergic Activity
3.2.2. Crude Extracts from Marine Animals as Potential Sources with Anti-Allergic Activity
3.3. Marine Microorganisms
Natural Products Derived from Marine Microorganisms with Anti-Allergic Activity
| Source of Compounds | The Sources of Isolation | Number of Compounds | Range of Dosage | Structure Type | Test System | Targets/Pathway/Process Mechanism | Reference |
|---|---|---|---|---|---|---|---|
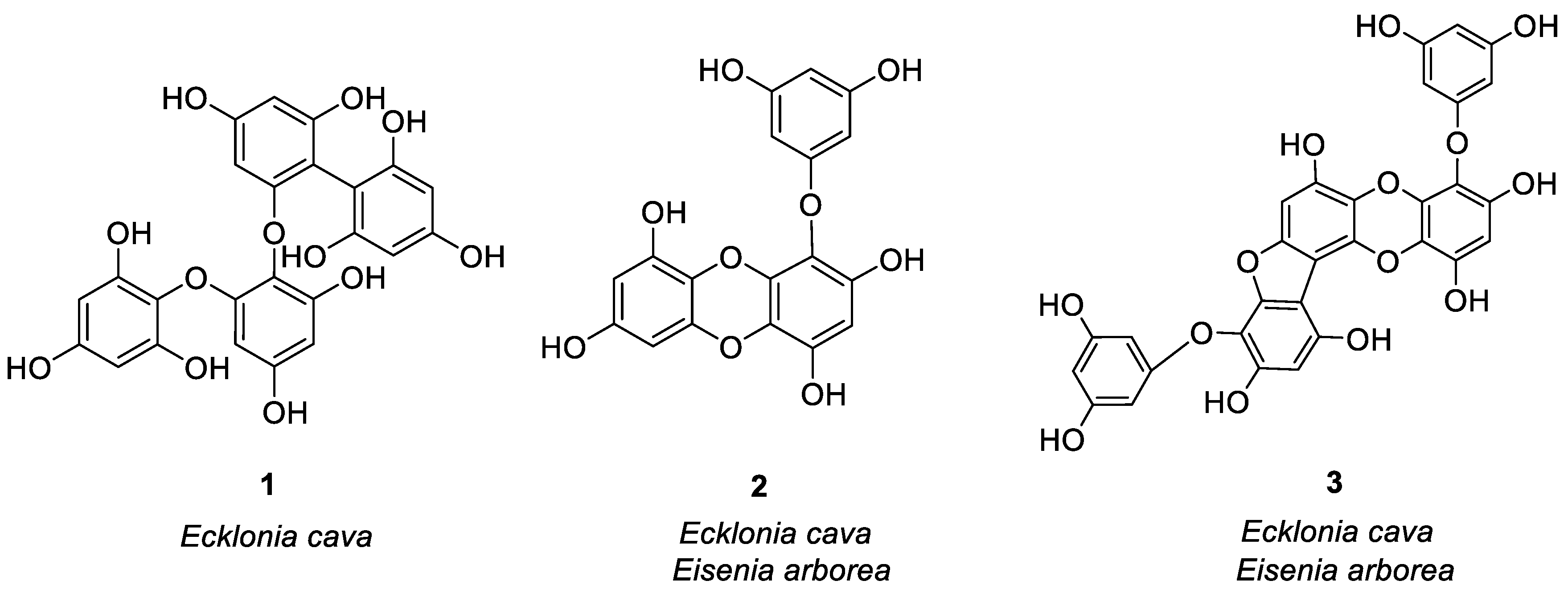
| Marine Plants | Ecklonia cava | Compound 1–3 | 100 μM | Polyphenol | Human basophilic KU812F cells and RBL-2H3 cells | FcεRI and IgE binding activity, histamine release, degranulation of cell | [25] |
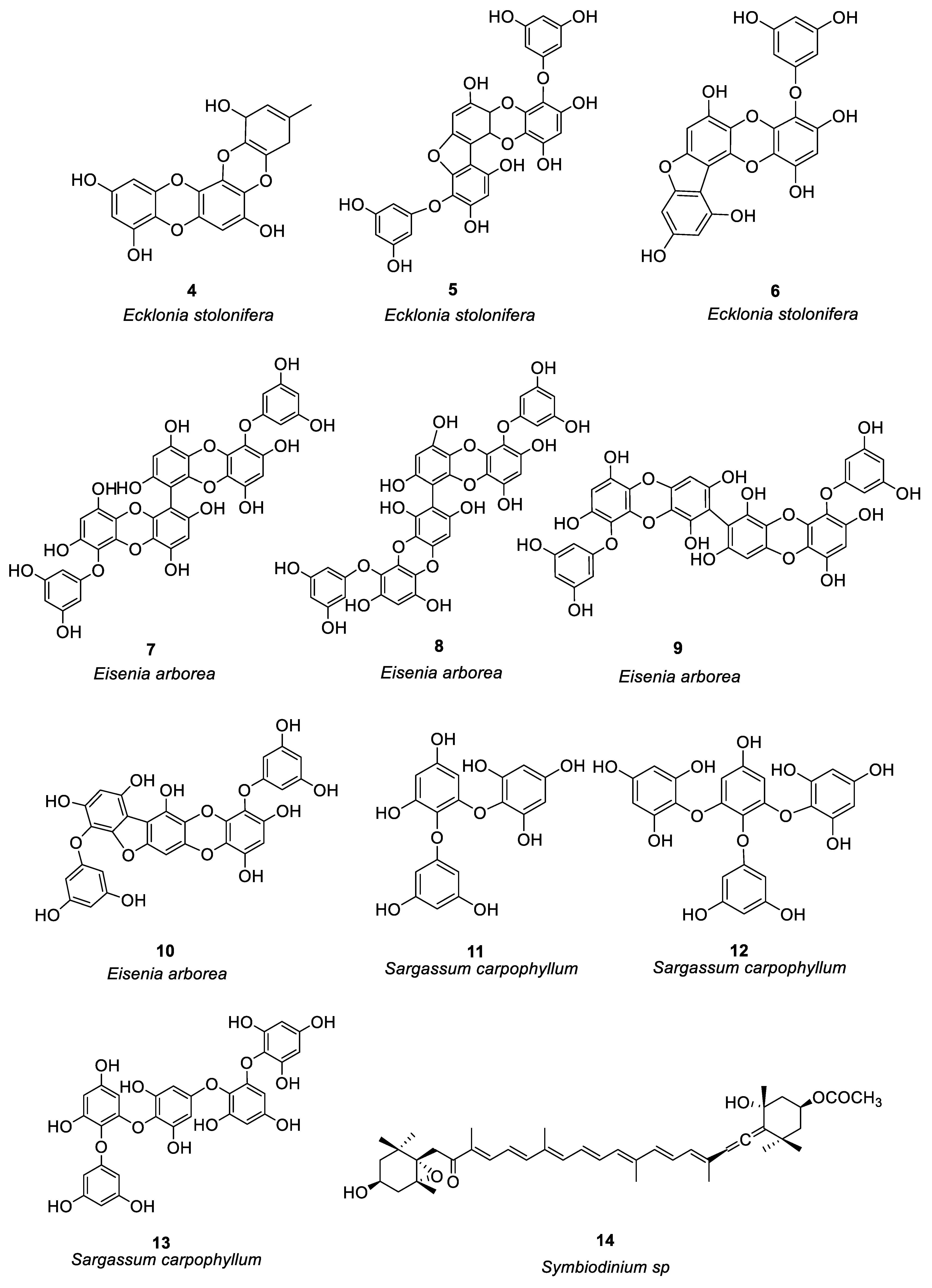
| Ecklonia stolonifera | Compound 4–5 | 50 μM | Polyphenol | Human basophilic KU812F cells | The expression of FcεRI, intracellular Ca2+ | [27] | |
| Ecklonia stolonifera Okamura | Compound 6 | 50 μM | Polyphenol | RBL-2H3 mast cell | Ca2+ concentration, mast cell degranulation, histamine release | [28] | |
| Eisenia arborea | Compound 2,3,7–10 | 10–200 µM | Polyphenol | DNP-BSA-induced RBL-2H3 mast cell | Release of histamine, leukotriene B4 and prostaglandin E2, H1 receptor | [29] | |
| Sargassum carpophyllum | Compound 11–13 | 40 μM | Polyphenol | DNP-HSA-induced RBL-2H3 cells | Release of β-hexosaminidase, mast cell degranulation | [31] | |
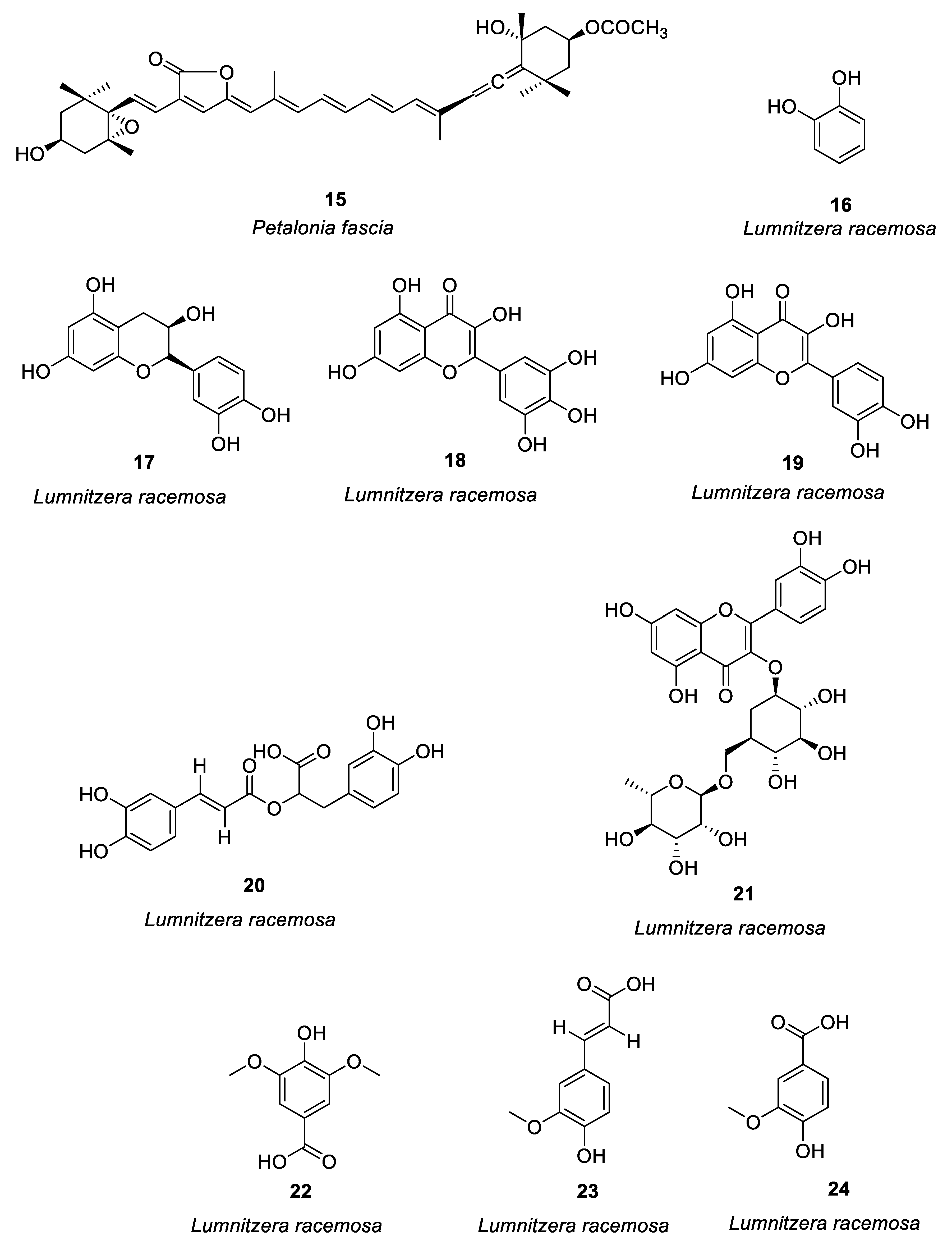
| Symbiodinium sp., Petalonia fascia | Compound 14–15 | 50 μg | Carotenoid | BALB/cAJc1 mice | Migration of eosinophils | [32] | |
| Lumnitzera racemosa | Compound 16–24 | / | (Ethanol extract) | Toluene 2,4-diisocyanate (TDI)-induced allergic model mice | IgE | [46] | |
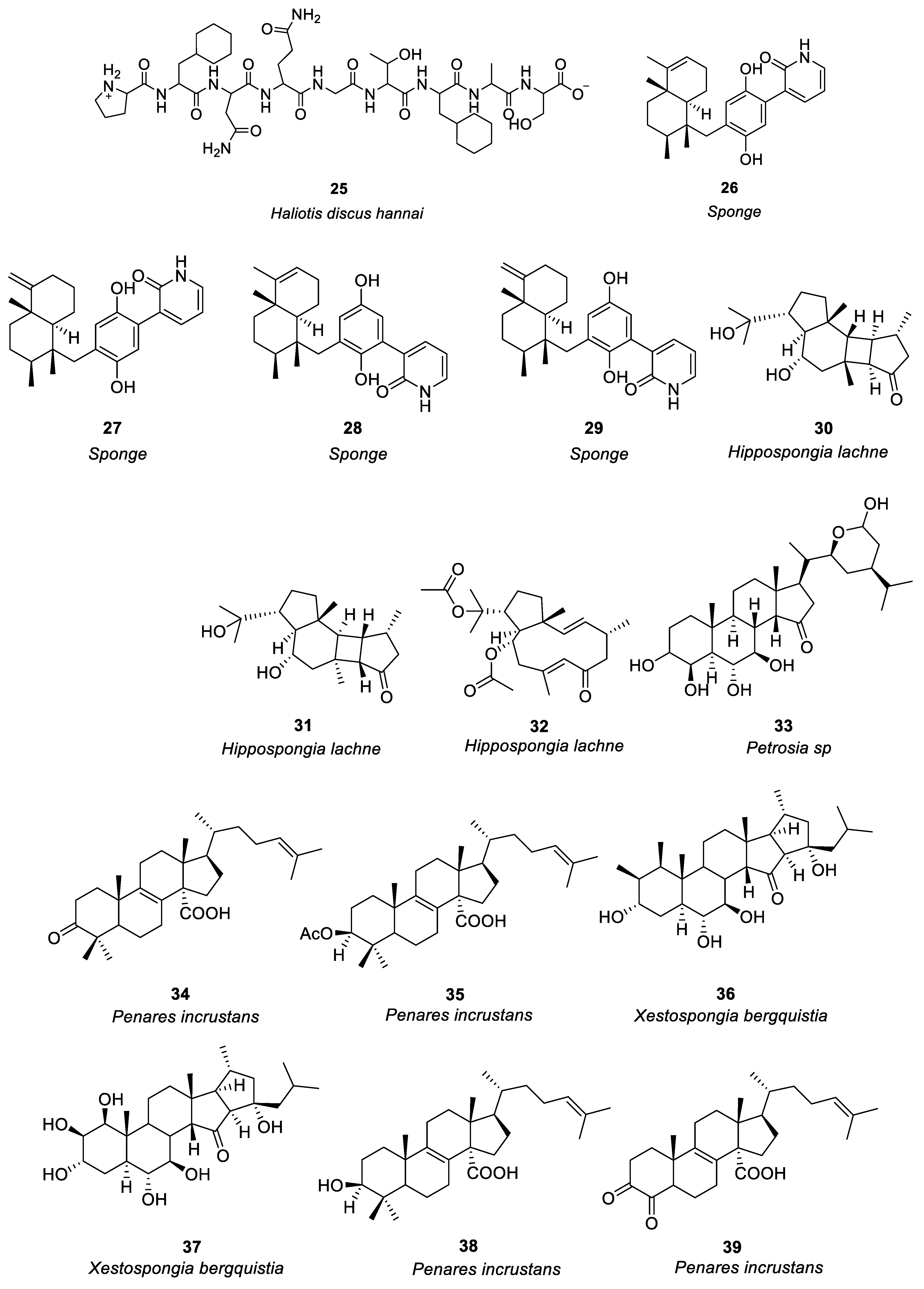
| Marine Animals | Haliotis discus hannai | Compound 25 | 50 mg/kg | Polypeptide | Passive cutaneous anaphylaxis in mice | Histamine release, FcεRI and IgE binding activity | [48] |
| Sponge | Compound 26–29 | 250 μg/mL | Terpenoids | RBL-2H3 mast cells | β-hexosaminidase, Syk/PLCγ-1, mast cell degranulation | [49] | |
| Hippospongia lachne | Compound 30–32 | 200 μg/mL | (Ethanol extract) | IgE-stimulated RBL-2H3 cells | β-hexosaminidase | [50] | |
| Petrosia sp. | Compound 33 | 3–30 μM | Sterol | OVA-induced mice | Histamine release levels | [51] | |
| Penares incrustans | Compound 34–35 | 0–10 μM | Triterpenoids | Anti-IgE-induced mast cells | Histamine release | [52] | |
| Xestospongia bergquistia, Penares incrustans | Compound 36–39 | 100 μM | Terpenoids | Anti-IgE-induced male Wistar rats’ mast cells | IP3 production, Histamine release, intracellular Ca2+, PLA2 | [53,54] | |
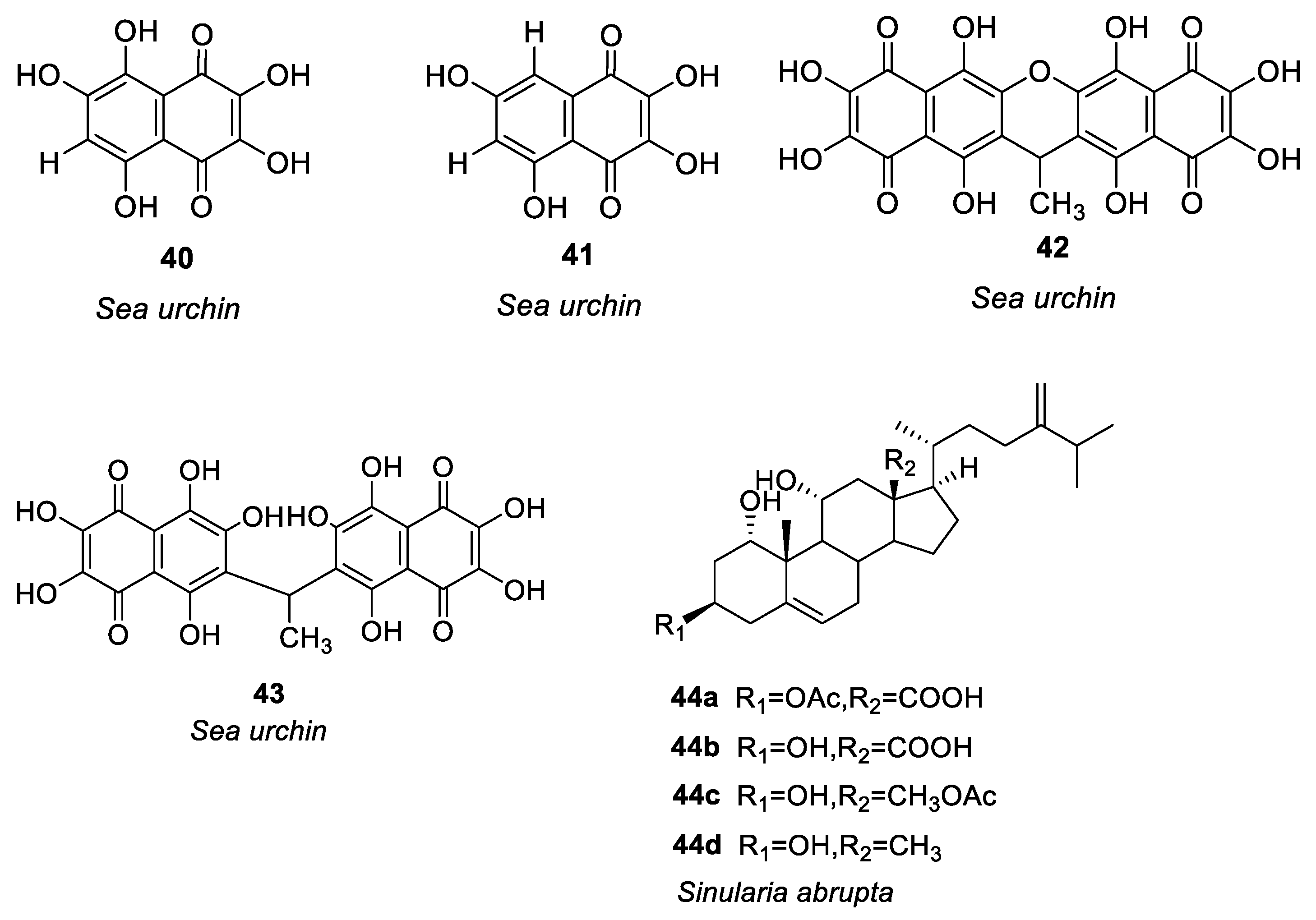
| Green sea urchin | Compound 40–43 | 1.2 μg/mL | Polyhydroxy-1,4-naphthoquinone | Histamine-induced guinea pigs | β-hexosaminidase | [55] | |
| Sinularia abrupta | Compound 44 | 0.04–1.5 μM | Polyhydroxysteroid | Anti-IgE-induced mice | Mast cell, histamine release | [57] | |
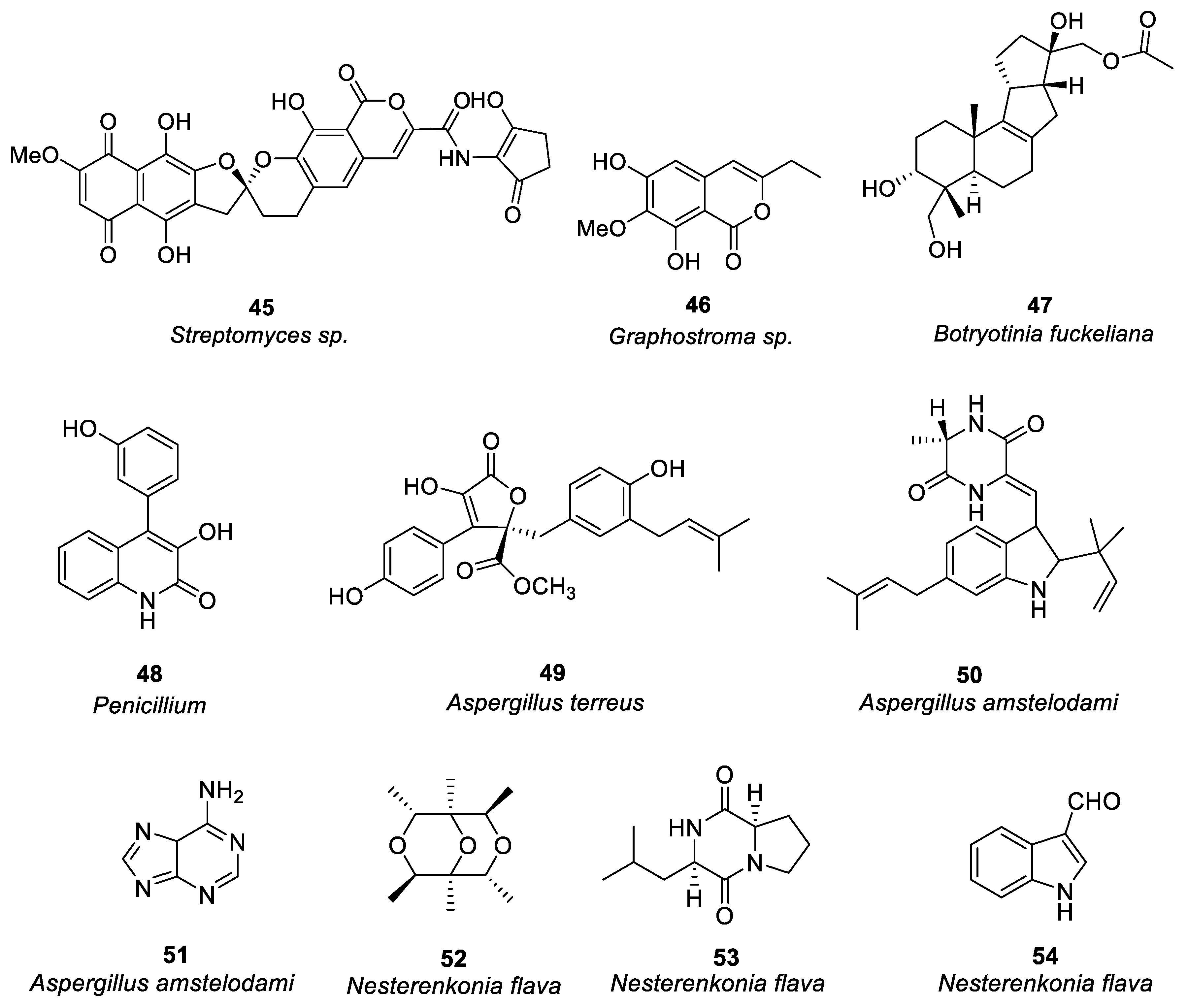
| Marine Microorganisms | Streptomyces sp. | Compound 45 | / | Macrolide | / | Mast cell degranulation, hyaluronidase | [62] |
| Graphostroma sp. Botryotinia fuckeliana | Compound 46–47 | 0–200 μM | Tetracyclic diterpenoids | RBL-2H3 cells | Histamine release, mast cell degranulation | [63,64] | |
| Penicillium | Compound 48 | 20 mg/kg | Quinoline alkaloid | OVA-induced RBL-2H3 cells | β-hexose and histamine, mast cell degranulation, IgE | [65] | |
| Aspergillus terreus | Compound 49 | 100 μM | Hemiterpenes | RBL-2H3 cells | β-hexosaminidase, IgE | [66] | |
| Aspergillus amstelodami | Compound 50–51 | 100 μM | β-lactams, adenine | RBL-2H3 cells | β-hexosaminidase | [67] | |
| Nesterenkonia flava | Compound 52–54 | 1.0–80.0 µg/mL | Cycloethers, diketopiperazine, alkaloid | RBL-2H3 cells | IgE, β-hexosaminidase | [68] |
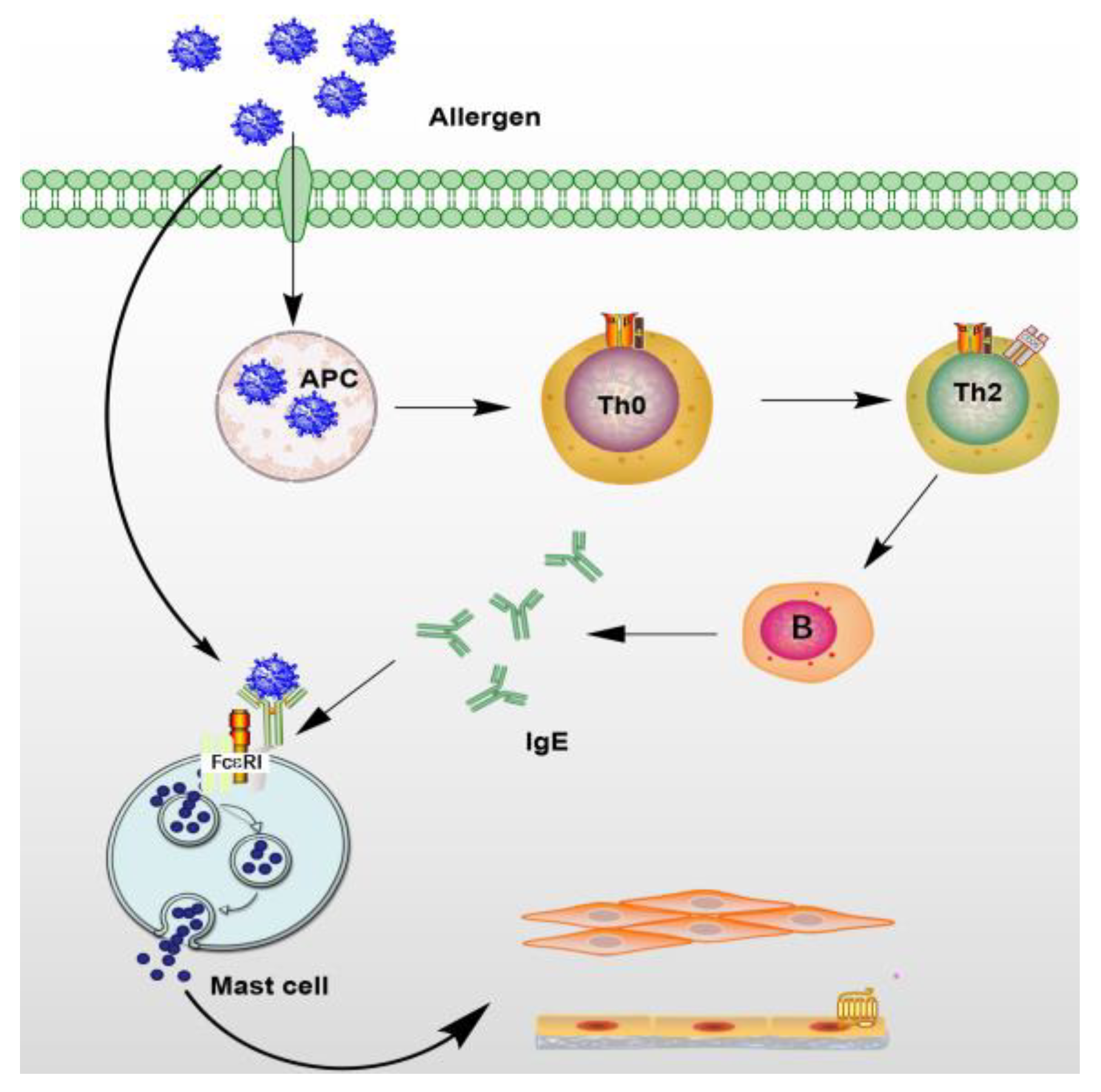
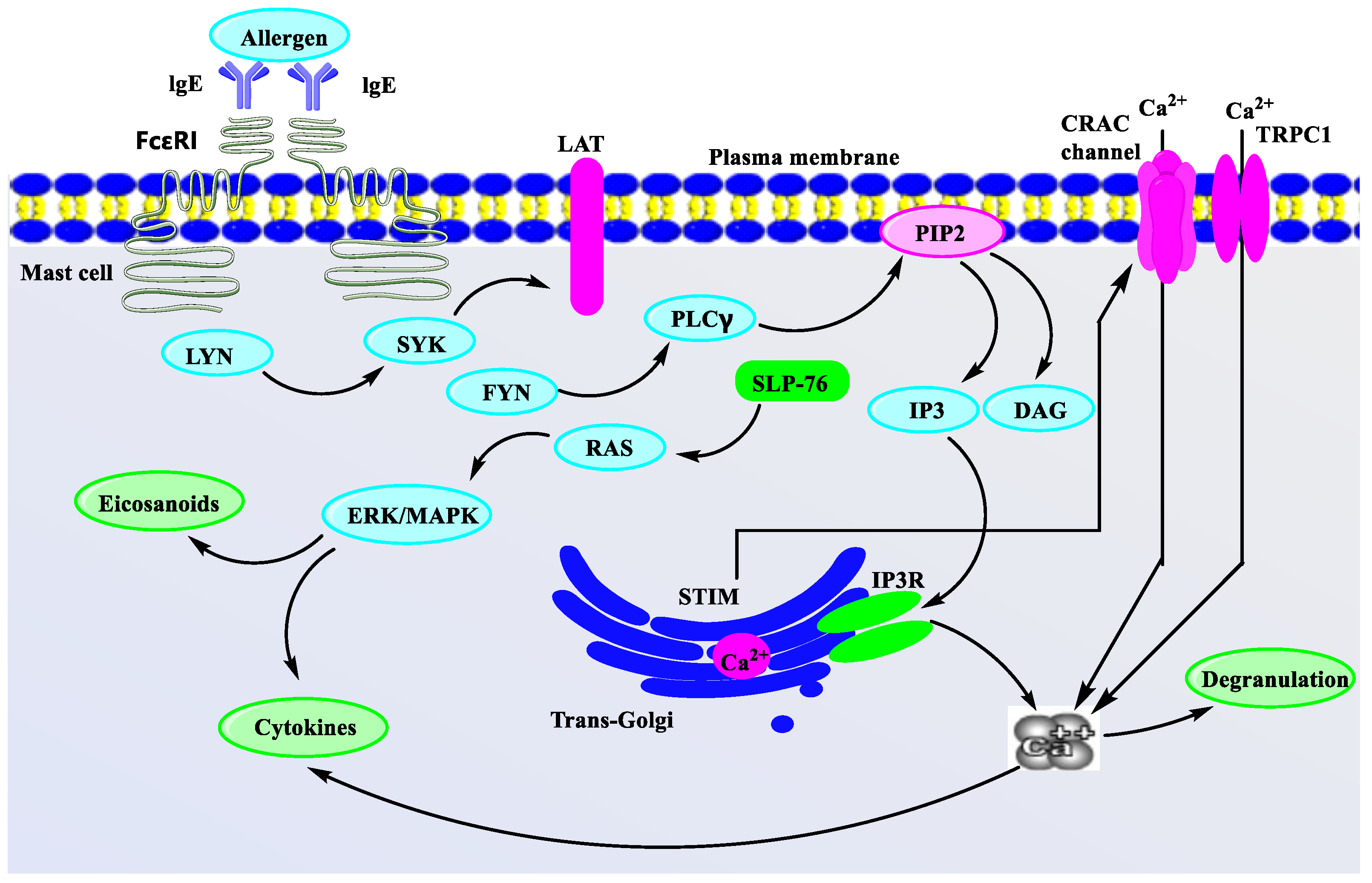
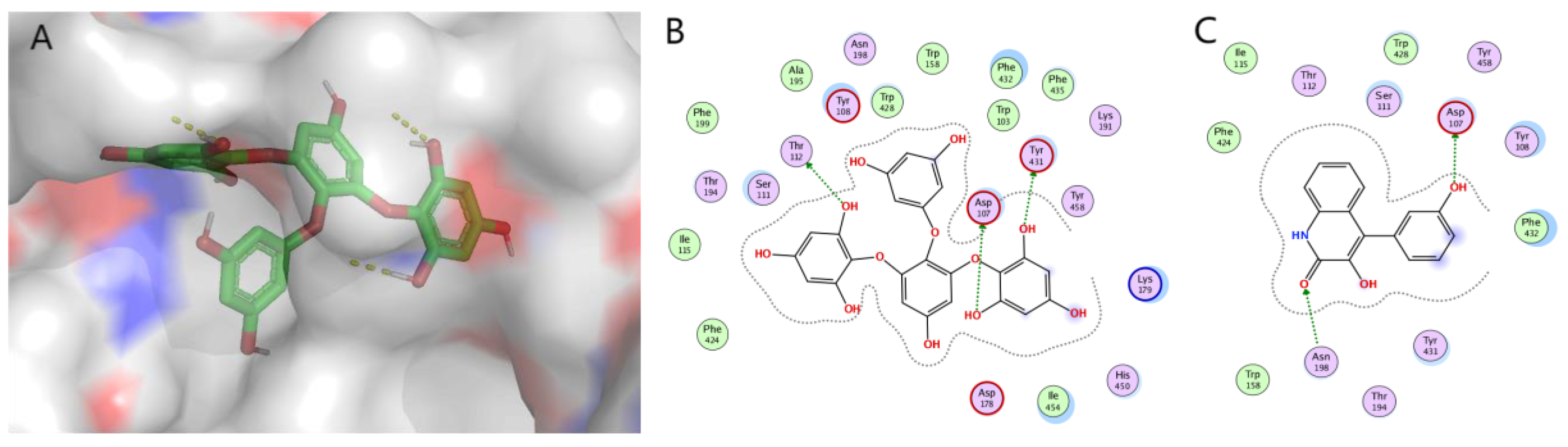
4. Potential Mechanism Study of Representative Natural Products
5. Discussion and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, S.Y.; Song, W.J.; Cho, S.H.; Chang, Y.S. Time trends of the prevalence of allergic diseases in Korea: A systematic literature review. Asia Pac. Allergy 2018, 8, e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oztop, N.; Demir, S.; Unal, D.; Beyaz, S.; Terzioglu, K.; Olgac, M.; Gelincik, A. Predictive factors of recurrence after omalizumab cessationvo in the elderly with urticaria: A real-life study. Allergy Asthma Proc. 2022, 43, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Fried, A.J.; Oettgen, H.C. Anti-IgE in the treatment of allergic disorders in pediatrics. Curr. Opin. Pediatr. 2010, 22, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Freidhoff, L.R.; Marsh, D.G. Relationship among Asthma, Serum IgE Levels and Skin Test Sensitivity to Inhaled Allergens. Int. Arch. Allergy Immunol. 2009, 100, 355–361. [Google Scholar] [CrossRef]
- Platts-Mills, T.A. The allergy epidemics: 1870–2010. J. Allergy Clin. Immunol. 2015, 136, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Sheikh, A.; Strachan, D.P.; Anderson, H.R. Time Trends in allergic disorders in the UK. Thorax 2007, 62, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prescott, S.; Nowak-Węgrzyn, A. Strategies to prevent or reduce allergic disease. Ann. Nutr. Metab. 2011, 59 (Suppl. S1), 28–42. [Google Scholar] [CrossRef] [Green Version]
- Imura, H.; Chen, J.; Kaneko, S.; Matsumoto, T. Analysis of Industrialization, Urbanization and Land-use Change in East Asia According to the DPSER Framework. In Proceedings of the 1999 NIES Workshop on Information Bases and Modeling for Land-Use and Land-Cover Changes Studies in East Asia; Kyushu University: Fukuoka City, Japan, 1999. [Google Scholar]
- Zhao, J.; Bai, J.; Shen, K.; Xiang, L.; Huang, S.; Chen, A.; Huang, Y.; Wang, J.; Ye, R. Self-reported prevalence of childhood allergic diseases in three cities of China: A multicenter study. BMC Public Health 2010, 10, 551. [Google Scholar] [CrossRef] [Green Version]
- Croom. Current Review of Allergic Diseases. Clin. Exp. Allergy 1999, 29, 1433. [CrossRef]
- Nielsen, D.; Dahl, R. Comparison of intranasal corticosteroids and antihistamines in allergic rhinitis: A review of randomized, controlled trials. Am. J. Respir. Med. 2003, 2, 55–65. [Google Scholar] [CrossRef]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Milia’n, E.; Dıáz, A.M. Allergy to house dust mites and asthma. P. R. Health Sci. J. 2004, 23, 47–57. [Google Scholar]
- Chen, B.; Laws, E.A. Is there a difference of temperature sensitivity between marine phytoplankton and heterotrophs? Limnol. Oceanogr. 2016, 62, 806–817. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 8–53. [Google Scholar] [CrossRef]
- Carté, B. Biomedical Potential of Marine Natural Products. Bioscience 1996, 46, 271–286. [Google Scholar]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and its anti-allergic immune response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro-Filho, J.; Piuvezam, M.R.; Bozza, P.T. Anti-allergic properties of curine, a bisbenzylisoquinoline alkaloid. Molecules 2015, 20, 4695–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pye, C.R.; Bertin, M.J.; Lokey, R.S.; Gerwick, W.H.; Linington, R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA 2017, 114, 5601–5606. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, L.J.; Malin, G.; Liss, P.S.; Küpper, F.C. Novel biogenic iodine-containing trihalomethanes and other short-lived halocarbons in the coastal East Atlantic. Glob. Biogeochem. Cycles 2000, 14, 1191–1204. [Google Scholar] [CrossRef]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Anis, M.; Ahmed, S.; Hasan, M.M. Algae as nutrition, medicine and cosmetic: The forgotten history, present status and future trends. World J. Pharm. Pharm. Sci. 2017, 6, 1934–1959. [Google Scholar]
- Laura, B. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 98, 317–333. [Google Scholar]
- Khuanjing, T.; Ongnok, B.; Maneechote, C.; Siri-Angkul, N.; Prathumsap, N.; Arinno, A.; Chunchai, T.; Arunsak, B.; Chat-tipakorn, S.C.; Chattipakorn, N. Acetylcholinesterase inhibitor ameliorates doxorubicin-induced cardiotoxicity through reducing RIP1-mediated necroptosis. Pharmacol. Res. 2021, 173, 105882. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, S.-H.; Le, Q.-T.; Kim, M.-M.; Kim, S.-K. Anti-allergic Effects of Phlorotannins on Histamine Release via Binding Inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Kim, H.S.; Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Jeon, Y.J.; Jee, Y.; Lee, J.; Kim, T.; Shim, S.Y.; Ahn, G. Eckol from Ecklonia cava Suppresses Immunoglobulin E-mediated Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Nutrients 2020, 12, 1361. [Google Scholar] [CrossRef]
- Shim, S.-Y.; Choi, J.-S.; Byun, D.-S. Inhibitory effects of phloroglucinol derivatives isolated from Ecklonia stolonifera on FcεRI expression. Bioorganic Med. Chem. 2009, 17, 4734–4739. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.-K.; Ryu, B.; Ngo, D.; Yoon, N.-Y.; Bach, L.G.; Hang, N.T.N. The Suppressive Activity of Fucofuroeckol-A Derived from Brown Algal Ecklonia stolonifera Okamura on UVB-Induced Mast Cell Degranulation. Mar. Drugs 2018, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Kakinuma, M.; Amano, H.; Miyata, M. Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cyclooxygenase-2 Signaling to Alleviate Mouse Ear Swelling. Mar. Drugs 2018, 16, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singha, B.; Shankarb, S.; Srivastavaa, R. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical Biochemical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, T.; Ito, C.; Itoigawa, M.; Shibata, T. Three phlorotannins from Sargassum carpophyllum are effective against the secretion of allergic mediators from antigen-stimulated rat basophilic leukemia cells. Food Chem. 2022, 377, 131992. [Google Scholar] [CrossRef]
- Onodera, K.-I.; Konishi, Y.; Taguchi, T.; Kiyoto, S.; Tominaga, A. Peridinin from the Marine Symbiotic Dinoflagellate, Symbiodinium sp., Regulates Eosinophilia in Mice. Mar. Drugs 2014, 12, 1773–1787. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.-R.; Hsu, K.-T.; Li, T.-L.; Chan, Y.-L.; Wu, C.-J. Topical application of fucoidan derived from Cladosiphon okamuranus alleviates atopic dermatitis symptoms through immunomodulation. Int. Immunopharmacol. 2021, 101, 108362. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-R.; Hsu, K.-T.; Hsu, W.-H.; Lee, B.-H.; Li, T.-L.; Chan, Y.-L.; Wu, C.-J. Immunomodulation and mechanisms of fucoidan from Cladosiphon okamuranus ameliorates atopic dermatitis symptoms. Int. J. Biol. Macromol. 2021, 189, 537–543. [Google Scholar] [CrossRef]
- Yu, B.; Bi, D.; Yao, L.; Li, T.; Gu, L.; Xu, H.; Li, X.; Li, H.; Hu, Z.; Xu, X. The inhibitory activity of alginate against allergic reactions in an ovalbumin-induced mouse model. Food Funct. 2020, 11, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Cao, M.; Pan, T.; Yang, Y.; Mao, H.; Sun, L.; Liu, G. Anti-allergic activity of R-phycocyanin from Porphyra haitanensis in antigen-sensitized mice and mast cells. Int. Immunopharmacol. 2015, 25, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Ngo, D.-H.; Kang, K.-H.; Park, S.-J.; Kim, S.-K. The role of peptides derived from Spirulina maximain downregulation of FcεRI-mediated allergic responses. Mol. Nutr. Food Res. 2014, 58, 2226–2234. [Google Scholar] [CrossRef]
- Kim, S.-K.; Lee, D.-Y.; Jung, W.-K.; Kim, J.-H.; Choi, I.; Park, S.-G.; Seo, S.-K.; Lee, S.-W.; Lee, C.M.; Yea, S.S.; et al. Effects of Ecklonia cava ethanolic extracts on airway hyperresponsiveness and inflammation in a murine asthma model: Role of suppressor of cytokine signaling. Biomed. Pharmacother. 2008, 62, 289–296. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, H.-S.; Sanjeewa, K.K.A.; Jung, K.; Jee, Y.; Jeon, Y.-J.; Fernando, I.P.S.; Ahn, G. Sargassum horneri as a Functional Food Ameliorated IgE/BSA-Induced Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Mar. Drugs 2020, 18, 594. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Kim, H.J.; Mihindukulasooriya, S.P.; Kim, A.; Kim, H.J.; Jeon, Y.-J.; Jee, Y. Sargassum horneri extract containing mojabanchromanol attenuates the particulate matter exacerbated allergic asthma through reduction of Th2 and Th17 response in mice. Environ. Pollut. 2020, 265 Pt B, 114094. [Google Scholar] [CrossRef]
- Jung, W.-K.; Choi, I.; Oh, S.; Park, S.-G.; Seo, S.-K.; Lee, S.-W.; Lee, D.-S.; Heo, S.-J.; Jeon, Y.-J.; Je, J.-Y.; et al. Anti-asthmatic effect of marine red alga (Laurencia undulata) polyphenolic extracts in a murine model of asthma. Food Chem. Toxicol. 2009, 47, 293–297. [Google Scholar] [CrossRef]
- Shi, C.; Pan, T.; Cao, M.; Liu, Q.; Zhang, L.; Liu, G. Suppression of Th2 immune responses by the sulfated polysaccharide from Porphyra haitanensis in tropomyosin-sensitized mice. Int. Immunopharmacol. 2015, 24, 211–218. [Google Scholar] [CrossRef]
- Han, J.; Liu, B.; Liu, Q.-M.; Zhang, Y.-F.; Liu, Y.-X.; Liu, H.; Cao, M.-J.; Liu, G.-M. Red Algae Sulfated Polysaccharides Effervescent Tablets Attenuated Ovalbumin-Induced Anaphylaxis by Upregulating Regulatory T cells in Mouse Models. J. Agric. Food Chem. 2019, 67, 11911–11921. [Google Scholar] [CrossRef]
- Raman, B.V.; Rao, D.N.; Radhakrishnan, T.M. Enteromorpha compressa (L.) Grevillean edible green alga as a source of antiallergic principle (S). J. Clin. Biochem. 2004, 19, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.S.; Park, W.S.; Heo, S.J.; Cha, S.H.; Kim, D.; Jeon, Y.J.; Park, S.G.; Seo, S.K.; Choi, J.S.; Park, S.J.; et al. Polyopes affinis alleviates airway inflammation in a murine model of allergic asthma. J. Biosci. 2011, 36, 869–877. [Google Scholar] [CrossRef]
- Acharyya, R.N.; Mithila, S.; Rani, S.; Islam, A.; Golder, M.; Ahmed, K.S.; Hossain, H.; Dev, S.; Das, A.K. Anti-allergic and Anti-hyperglycemic Potentials of Lumnitzera racemose Leaves: In vivo and In silico Studies. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2022, 1–12. [Google Scholar] [CrossRef]
- Pita, L.; Rix, L.; Slaby, B.M.; Franke, A.; Hentschel, U. The sponge holobiont in a changing ocean: From microbes to ecosystems. Microbiome 2018, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-C.; Lee, D.-S.; Park, W.S.; Yoo, J.S.; Yim, M.-J.; Qian, Z.-J.; Lee, C.-M.; Oh, J.; Jung, W.-K.; Choi, I.-W. Anti-allergic effects of a nonameric peptide isolated from the intestine gastrointestinal digests of abalone (Haliotis discus hannai) in activated HMC-1 human mast cells. Int. J. Mol. Med. 2015, 37, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Jiao, W.-H.; Cheng, B.-H.; Shi, G.-H.; Chen, G.-D.; Gu, B.-B.; Zhou, Y.-J.; Hong, L.-L.; Yang, F.; Liu, Z.-Q.; Qiu, S.-Q.; et al. Dysivillosins A–D, Unusual Anti-allergic Meroterpenoids from the Marine Sponge Dysidea villosa. Sci. Rep. 2017, 7, 8947. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.-L.; Yu, H.-B.; Wang, J.; Jiao, W.-H.; Cheng, B.-H.; Yang, F.; Zhou, Y.-J.; Gu, B.-B.; Song, S.-J.; Lin, H.-W. Unusual Anti-allergic Diterpenoids from the Marine Sponge Hippospongia lachne. Sci. Rep. 2017, 7, 43138. [Google Scholar] [CrossRef] [Green Version]
- Bramley, A.M.; Langlands, J.M.; Jones, A.K.; Burgoyne, D.L.; Li, Y.; Andersen, R.J.; Salari, H. Effects of IZP-94005 (contignasterol) on antigen-induced bronchial responsiveness in ovalbumin-sensitized guinea-pigs. Br. J. Pharmacol. 1995, 115, 1433. [Google Scholar] [CrossRef] [Green Version]
- Shoji, N.; Umeyama, A.; Motoki, S.; Arihara, S.; Ishida, T.; Nomoto, K.; Kobayashi, J.; Takei, M. Potent Inhibitors of Histamine Release, Two Novel Triterpenoids from the Okinawan Marine Sponge Penares incrustans. J. Nat. Prod. 1992, 55, 1682–1685. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Umeyama, A.; Shoji, N.; Arihara, S.; Endo, K. Mechanism of inhibition of IgE-dependent histamine release from rat mast cells by xestobergsterol A from the Okinawan marine spongeXestospongia bergquistia. Cell Mol. Life Sci. 1993, 49, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Umeyama, A.; Shoji, N.; Arihara, S.; Endo, K. Mechanism of Inhibition of IgE-Dependent Histamine Release from Rat Mast Cells by Penasterol and Penasterone. J. Pharm. Sci. 1995, 84, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Shikov, A.N.; Makarova, M.N.; Ivanova, S.A.; Kosman, V.M.; Makarov, V.G.; Bazgier, V.; Berka, K.; Otyepka, M.; Ulrichová, J. Antiallergic Effects of Pigments Isolated from Green Sea Urchin (Strongylocentrotus droebachiensis) Shells. Planta Medica 2013, 79, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T.; Li, Y.; Guo, Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Shoji, N.; Umeyama, A.; Takei, M.; Arihara, S. Potent inhibitors of histamine release: Polyhydroxylated sterols from the okinawan soft coral Sinularia abrupta. J. Pharm. Sci. 1994, 83, 761–762. [Google Scholar] [CrossRef]
- Kim, T.-H.; Heo, S.-Y.; Oh, G.-W.; Park, W.S.; Choi, I.-W.; Qian, Z.-J.; Jung, W.-K. Anti-Allergic Effect of Low Molecular Weight Digest from Abalone Viscera on Atopic Dermatitis-Induced NC/Nga. Mar. Drugs 2021, 19, 634. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-I.; Kang, S.A.; Anisuzzaman; Jeong, U.-C.; Jin, F.; Kang, S.-J.; Lee, J.-Y.; Yu, H.S. Sea Cucumber Lipid-Soluble Extra Fraction Prevents Ovalbumin-Induced Allergic Airway Inflammation. J. Med. Food 2018, 21, 21–29. [Google Scholar] [CrossRef]
- Willemsen, L.E. Dietary n-3 long chain polyunsaturated fatty acids in allergy prevention and asthma treatment. Eur. J. Pharmacol. 2016, 785, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Aryani, A.; Suprayitno, E.; Sasmito, B.B.; Hardoko, H. Characterization and identification of charcoal of inedible Kerandang fish (Channa pleurophthalmus Blkr) body parts and potential antiallergenic properties. Veter World 2020, 13, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Harunari, E.; Imada, C.; Igarashi, Y.; Fukuda, T.; Terahara, T.; Kobayashi, T. Hyaluromycin, a new hyaluronidase inhibitor of polyketide origin from marine Streptomyces sp. Mar. Drugs 2014, 12, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Liu, Q.; Xia, J.-M.; Xie, C.-L.; Luo, Z.-H.; Shao, Z.; Liu, G.-M.; Yang, X.-W. Polyketides from the Deep-Sea-Derived Fungus Graphostroma sp. MCCC 3A00421 Showed Potent Antifood Allergic Activities. J. Agric. Food Chem. 2018, 66, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Xie, C.L.; Xia, J.M.; Liu, Q.M.; Peng, G.; Liu, G.M.; Yang, X.W.; Botryotins, A.-H. Tetracyclic Diterpenoids Representing Three Carbon Skeletons from a Deep-Sea-Derived Botryotinia fuckeliana. Org. Lett. 2020, 22, 580–583. [Google Scholar] [CrossRef]
- Shu, Z.; Liu, Q.; Xing, C.; Zhang, Y.; Zhou, Y.; Zhang, J.; Liu, H.; Cao, M.; Yang, X.; Liu, G. Viridicatol Isolated from Deep-Sea Penicillium Griseofulvum Alleviates Anaphylaxis and Repairs the Intestinal Barrier in Mice by Suppressing Mast Cell Activation. Mar. Drugs 2020, 18, 517. [Google Scholar] [CrossRef]
- Uras, I.S.; Ebada, S.S.; Korinek, M.; Albohy, A.; Abdulrazik, B.S.; Wang, Y.H.; Chen, B.H.; Horng, J.T.; Lin, W.; Hwang, T.L.; et al. Anti-Inflammatory, Antiallergic, and COVID-19 Main Protease (M(pro)) Inhibitory Activities of Butenolides from a Marine-Derived Fungus Aspergillus terreus. Molecules 2021, 26, 3354. [Google Scholar] [CrossRef]
- Elsbaey, M.; Sallam, A.; El-Metwally, M.; Nagata, M.; Tanaka, C.; Shimizu, K.; Miyamoto, T. Melanogenesis Inhibitors from the Endophytic Fungus Aspergillus amstelodami. Chem. Biodivers 2019, 16, e1900237. [Google Scholar] [CrossRef]
- Xie, C.-L.; Liu, Q.; Xia, J.-M.; Gao, Y.; Yang, Q.; Shao, Z.-Z.; Liu, G.; Yang, X.-W. Anti-Allergic Compounds from the Deep-Sea-Derived Actinomycete Nesterenkonia flava MCCC 1K00610. Mar. Drugs 2017, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hourihane, J.O.B.; Allen, K.J.; Shreffler, W.G.; Dunngalvin, G.; Nordlee, J.A.; Zurzolo, G.A.; Dunngalvin, A.; Gurrin, L.C.; Baumert, J.L.; Taylor, S.L. Peanut Allergen Threshold Study (PATS): Novel single-dose oral food challenge study to validate eliciting doses in children with peanut allergy. J. Allergy Clin. Immunol. 2017, 5, 1583–1590. [Google Scholar] [CrossRef] [Green Version]
- Rosenstreich, D.L.; Eggleston, P.; Kattan, M.; Baker, D.; Slavin, R.G.; Gergen, P.; Mitchell, H.; McNiff-Mortimer, K.; Lynn, H.; Ownby, D.; et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N. Engl. J. Med. 1997, 336, 1356–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.Z.; Qin, X.J. CD4+CD25+ regulatory T lymphocytes in allergy and asthma. Allergy 2005, 60, 986–995. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, H.J.; Sutton, B.J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.M.; Kepley, C.L.; Ortega, E.; Wilson, B.S. Immunologically mediated signaling in basophils and mast cells: Finding therapeutic targets for allergic diseases in the human FcεR1 signaling pathway. Immunopharmacology 2000, 48, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2011, 106, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breedveld, A.; Groot Kormelink, T.; van Egmond, M.; de Jong, E.C. Granulocytes as modulators of dendritic cell function. J. Leukoc. Biol. 2017, 102, 1003–1016. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.W.; Rahman, T.; Tovey, S.C.; Dedos, S.G.; Taylor, E.J.; Velamakanni, S. IP3 receptors: Some lessons from DT40 cells. Immunol. Rev. 2009, 231, 23–44. [Google Scholar] [CrossRef]
- Putney, J.W., Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Michell, R.H. Inositol phospholipids and cell surface receptor function. Biochim. Biophys. Acta Rev. Biomembr. 1975, 415, 81–147. [Google Scholar] [CrossRef]
- Liou, J.; Kim, M.L.; Do Heo, W.; Jones, J.T.; Myers, J.W.; Ferrell, J.E., Jr.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Putney, J.W. Capacitative calcium entry: From concept to molecules. Immunol. Rev. 2009, 231, 10–22. [Google Scholar] [CrossRef]
- Hartzell, C.A.; Jankowska, K.I.; Burkhardt, J.K.; Lewis, R.S. Calcium influx through CRAC channels controls actin organization and dynamics at the immune synapse. eLife 2016, 5, e14850. [Google Scholar] [CrossRef]
- Zhang, J.; Berenstein, E.; Siraganian, R.P. Phosphorylation of Tyr342 in the Linker Region of Syk Is Critical for FcεRI Signaling in Mast Cells. Mol. Cell Biol. 2002, 22, 8144–8154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svetlov, S.I.; Siafaka-Kapadai, A.; Hanahan, D.J.; Olson, M.S. Signaling responses to alkyllysophosphatidic acid: The activation of phospholipases A2 and C and protein tyrosine phosphorylation in human platelets. Arch. Biochem. Biophys. 1996, 336, 59. [Google Scholar] [CrossRef]
- Hagar, R.E.; Burgstahler, A.D.; Nathanson, M.H.; Ehrlich, B.E. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature 1998, 396, 81. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Sun, P.; Shi, J.; Wu, X.; Liu, C.; Peng, Z.; Han, H.; Xu, S.; Yang, Y.; et al. PCV2 trigger apoptosis of PK-15 cells through the PLC-IP3R-Ca2+ signaling pathway. Authorea Prepr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Chustz, R.T.; Ogasawara, T.; Ogasawara, T.; Kulka, M.; Saito, H.; Schleimer, R.P.; Matsumoto, K. Dexamethasone and FK506 Inhibit Expression of Distinct Subsets of Chemokines in Human Mast Cells. J. Immunol. 2009, 182, 7233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Rivera, J.; Fierro, N.A.; Olivera, A.; Suzuki, R. New insights on mast cell activation via the high affinity receptor for IgE. Adv. Immunol. 2008, 98, 85–120. [Google Scholar] [PubMed] [Green Version]
- Shilling, R.A.; Pinto, J.M.; Decker, D.C.; Schneider, D.H.; Bandukwala, H.S.; Schneider, J.R.; Camoretti-Mercado, B.; Ober, C.; Sperling, A.I. Cutting edge: Polymorphisms in the ICOS promoter region are associated with allergic sensitization and Th2 cytokine production. J. Immunol. 2005, 175, 2061–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Makhov, A.M.; Bear, J.E. F-actin binding is essential for coronin 1B function in vivo. J. Cell Sci. 2007, 120, 1779–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrendorff, N.; Dolai, S.; Hong, W.; Gaisano, H.Y.; Thorn, P. Vesicle-associated Membrane Protein 8 (VAMP8) Is a SNARE (Soluble N-Ethylmaleimide-sensitive Factor Attachment Protein Receptor) Selectively Required for Sequential Granule-to-granule Fusion. Commun. Integr. Biol. 2012, 286, 29627–29634. [Google Scholar] [CrossRef] [Green Version]
- Ohlig, S.; Farshi, P.; Pickhinke, U.; van den Boom, J.; Höing, S.; Jakuschev, S.; Hoffmann, D.; Dreier, R.; Schöler, H.R.; Dierker, T.; et al. Sonic Hedgehog Shedding Results in Functional Activation of the Solubilized Protein. Dev. Cell 2011, 20, 764–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudeck, J.; Froebel, J.; Kotrba, J.; Lehmann, C.H.K.; Dudziak, D.; Speier, S.; Nedospasov, S.A.; Schraven, B.; Dudeck, A. Engulfment of mast cell secretory granules on skin inflammation boosts dendritic cell migration and priming efficiency. J. Allergy Clin. Immunol. 2019, 143, 1849–1864.e4. [Google Scholar] [CrossRef]
- Akata, M.; Sabe, H.; Hata, A.; Inazu, T.; Homma, Y.; Nukada, T.; Yamamura, H.; Kurosaki, T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994, 13, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, R.L.; Desai, P.J.; Dunford, P.J.; Fung-Leung, W.-P.; Hofstra, C.L.; Jiang, W.; Nguyen, S.; Riley, J.P.; Sun, S.; Williams, K.N.; et al. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties, Pharmacol. Exp. Ther. 2004, 309, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Cowart, M.; Altenbach, R.; Liu, H.; Hsieh, G.; Drizin, I.; Milicic, I.; Miller, T.; Witte, D.; Wishart, N.; Fix-Stenzel, S. Rotationally constrained 2, 4-diamino-5, 6-disubstituted pyrimidines: A new class of histamine H4 receptor antagonists with improved druglikeness and in vivo efficacy in pain and inflammation models. J. Med. Chem. 2008, 51, 6547–6557. [Google Scholar] [CrossRef]
- Calderon, M.A.; Alves, B.; Jacobson, M.; Hurwitz, B.; Sheikh, A.; Durham, S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst. Rev. 2010, 5, 1279–1379. [Google Scholar] [CrossRef]
- Corren, J.; Manning, B.E.; Thompson, S.F.; Hennessy, S.; Strom, B.L. Rhinitis therapy and the prevention of hospital care for asthma: A case-control study. J. Allergy Clin. Immunol. 2004, 113, 415–419. [Google Scholar] [CrossRef]
- Ilja, C.W.A.; Peter, C.H.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S. [Google Scholar]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T.; Moitinho-Silva, L.; Lurgi, M.; Björk, J.R.; Easson, C.; Astudillo-García, C.; Olson, J.B.; Erwin, P.M.; López-Legentil, S.; Luter, H.; et al. Diversity, structure and convergent evolution of the global sponge microbiome. Nat. Commun. 2016, 7, 11870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamura, T.; Shiroishi, M.; Weyand, S.; Tsujimoto, H.; Winter, G.; Katritch, V.; Abagyan, R.; Cherezov, V.; Liu, W.; Han, G.W.; et al. Structure of the human histamine H1 receptor complex with doxepin. Nature 2011, 475, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Zhang, S.; Javeed, A.; Jian, C.; Liu, Y.; Sun, J.; Wu, S.; Fu, P.; Han, B. Structures and Anti-Allergic Activities of Natural Products from Marine Organisms. Mar. Drugs 2023, 21, 152. https://doi.org/10.3390/md21030152
Chen N, Zhang S, Javeed A, Jian C, Liu Y, Sun J, Wu S, Fu P, Han B. Structures and Anti-Allergic Activities of Natural Products from Marine Organisms. Marine Drugs. 2023; 21(3):152. https://doi.org/10.3390/md21030152
Chicago/Turabian StyleChen, Na, Shanshan Zhang, Ansar Javeed, Cuiqin Jian, Yi Liu, Jinlyu Sun, Shandong Wu, Peng Fu, and Bingnan Han. 2023. "Structures and Anti-Allergic Activities of Natural Products from Marine Organisms" Marine Drugs 21, no. 3: 152. https://doi.org/10.3390/md21030152







