Dietary Phospholipids Alleviate Diet-Induced Obesity in Mice: Which Fatty Acids and Which Polar Head
Abstract
:1. Introduction
2. Results
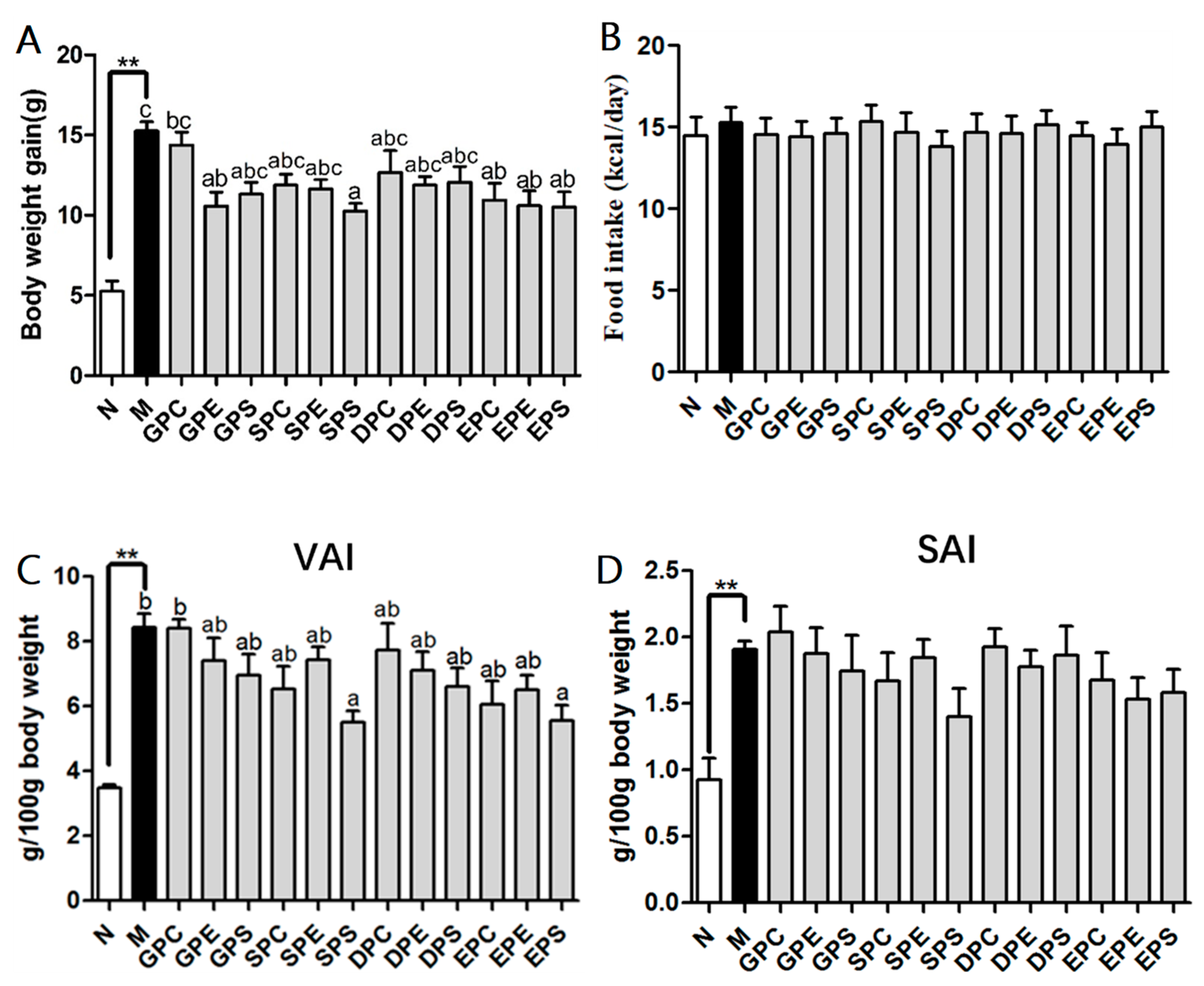
2.1. Effects of Different PLs on Growth Parameters in Mice
2.2. Effects of Different PLs on Lipid Profile in Serum of Mice
2.3. Effects of Different PLs on Inflammation Factors in Serum of Mice
2.4. Effects of Different PLs on mRNA and Protein Expression Associated with Lipid Metabolism in the Liver
2.5. Interaction Effects between Fatty Acids and Polar Head Groups
3. Discussion
4. Materials and Methods
4.1. Preparation of Phospholipids
4.2. Animals and Diets
4.3. Analysis of Serum Parameters
4.4. RNA Extraction and Quantitative Real Time PCR
4.5. Western Blot Analysis
4.6. Luciferase Reporter Assay
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and obesity: Prevalence, consequences, and causes of a growing public health problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Botchlett, R.; Woo, S.-L.; Liu, M.; Pei, Y.; Guo, X.; Li, H.; Wu, C. Nutritional approaches for managing obesity-associated metabolic diseases. J. Endocrinol. 2017, 233, R145–R171. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Lanaspa, M.A.; Sanchez-Lozada, L.G.; Tolan, D.; Nakagawa, T.; Ishimoto, T.; Andres-Hernando, A.; Rodriguez-Iturbe, B.; Stenvinkel, P. The fructose survival hypothesis for obesity. Philos. Trans. R. Soc. B 2023, 378, 20220230. [Google Scholar] [CrossRef] [PubMed]
- Bolling, C.F.; Armstrong, S.C.; Reichard, K.W.; Michalsky, M.P.; Haemer, M.A.; Muth, N.D.; Rausch, J.C.; Rogers, V.W.; Heiss, K.F.; Besner, G.E. Metabolic and bariatric surgery for pediatric patients with severe obesity. Pediatrics 2019, 144, e20193224. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Zingg, J.M.; Fassini, P.G.; Suen, V.M.M. Bioactive dietary components—Anti-obesity effects related to energy metabolism and inflammation. BioFactors 2023, 49, 297–321. [Google Scholar] [CrossRef]
- Kumar, M.; Kaushik, D.; Kaur, J.; Proestos, C.; Oz, F.; Oz, E.; Gupta, P.; Kundu, P.; Kaur, A.; Anisha, A. A critical review on obesity: Herbal approach, bioactive compounds, and their mechanism. Appl. Sci. 2022, 12, 8342. [Google Scholar] [CrossRef]
- Blesso, C.N. Egg phospholipids and cardiovascular health. Nutrients 2015, 7, 2731–2747. [Google Scholar] [CrossRef]
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.; Tandy, S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2010, 2, 116–127. [Google Scholar] [CrossRef]
- Dijkstra, A. Edible Oil Processing from a Patent Perspective—Refining. Degumming—Introduction; Springer: Boston, MA, USA, 2013; pp. 121–155. [Google Scholar]
- Wen, M.; Xu, J.; Ding, L.; Zhang, L.; Du, L.; Wang, J.; Wang, Y.; Xue, C. Eicosapentaenoic acid-enriched phospholipids improve Aβ1–40-induced cognitive deficiency in a rat model of Alzheimer’s disease. J. Funct. Foods 2016, 24, 537–548. [Google Scholar] [CrossRef]
- Gao, X.; Du, L.; Randell, E.; Zhang, H.; Li, K.; Li, D. Effect of different phosphatidylcholines on high fat diet-induced insulin resistance in mice. Food Funct. 2021, 12, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Shi, H.H.; Wang, C.C.; Wang, Y.M.; Wei, Z.H.; Xue, C.H.; Mao, X.Z.; Zhang, T.T. Targeted Lipidomics Reveal the Effects of Different Phospholipids on the Phospholipid Profiles of Hepatic Mitochondria and Endoplasmic Reticulum in High-Fat/High-Fructose-Diet-Induced Nonalcoholic Fatty Liver Disease Mice. J. Agric. Food Chem. 2022, 70, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Xu, J.; Wang, Y.M.; Xue, C.H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Han, B.H.; Kim, J.S.; Kim, K.; Shim, I. Neuroprotective effect of bean phosphatidylserine on TMT-induced memory deficits in a rat model. Int. J. Mol. Sci. 2020, 21, 4901. [Google Scholar] [CrossRef]
- Zhao, Y.-C.; Zhou, M.-M.; Zhang, L.-Y.; Cong, P.-X.; Xu, J.; Xue, C.-H.; Yanagita, T.; Chi, N.; Zhang, T.-T.; Liu, F.-H. Recovery of brain DHA-containing phosphatidylserine and ethanolamine plasmalogen after dietary DHA-enriched phosphatidylcholine and phosphatidylserine in SAMP8 mice fed with high-fat diet. Lipids Health Dis. 2020, 19, 104. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, T.; Che, H.; Zhang, L.; Xue, C.; Chang, Y.; Wang, Y. DHA-enriched phosphatidylcholine and DHA-enriched phosphatidylserine improve age-related lipid metabolic disorder through different metabolism in the senescence-accelerated mouse. Eur. J. Lipid Sci. Technol. 2018, 120, 1700490. [Google Scholar] [CrossRef]
- Liu, X.; Xue, Y.; Liu, C.; Lou, Q.; Wang, J.; Yanagita, T.; Xue, C.; Wang, Y. Eicosapentaenoic acid-enriched phospholipid ameliorates insulin resistance and lipid metabolism in diet-induced-obese mice. Lipids Health Dis. 2013, 12, 109. [Google Scholar] [CrossRef]
- Liu, X.; Cui, J.; Li, Z.; Xu, J.; Wang, J.; Xue, C.; Wang, Y. Comparative study of DHA-enriched phospholipids and EPA-enriched phospholipids on metabolic disorders in diet-induced-obese C57BL/6J mice. Eur. J. Lipid Sci. Technol. 2014, 116, 255–265. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.L.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef]
- Kersten, S. Integrated physiology and systems biology of PPARalpha. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef]
- Shirouchi, B.; Nagao, K.; Inoue, N.; Ohkubo, T.; Hibino, H.; Yanagita, T. Effect of dietary omega 3 phosphatidylcholine on obesity-related disorders in obese Otsuka Long-Evans Tokushima fatty rats. J. Agric. Food Chem. 2007, 55, 7170–7176. [Google Scholar] [CrossRef] [PubMed]
- Buang, Y.; Wang, Y.-M.; Cha, J.-Y.; Nagao, K.; Yanagita, T. Dietary phosphatidylcholine alleviates fatty liver induced by orotic acid. Nutrition 2005, 21, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, K.; Mawatari, K.; Murata, M.; Ikeda, I.; Sugano, M. The contrasting effect of dietary phosphatidylethanolamine and phosphatidylcholine on serum lipoproteins and liver lipids in rats. J. Nutr. 1983, 113, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.; Clement, K.; Baur, L.; Tordjman, J. Obesity and low-grade inflammation: A paediatric perspective. Obes. Rev. 2010, 11, 118–126. [Google Scholar] [CrossRef]
- King, G.L. The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef]
- Detopoulou, P.; Panagiotakos, D.B.; Antonopoulou, S.; Pitsavos, C.; Stefanadis, C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2008, 87, 424–430. [Google Scholar] [CrossRef]
- Sioriki, E.; Smith, T.K.; Demopoulos, C.A.; Zabetakis, I. Structure and cardioprotective activities of polar lipids of olive pomace, olive pomace-enriched fish feed and olive pomace fed gilthead sea bream (Sparus aurata). Food Res. Int. 2016, 83, 143–151. [Google Scholar] [CrossRef]
- Shirouchi, B.; Nagao, K.; Furuya, K.; Shiojiri, M.; Liu, X.; Yanagita, T. Physiological effects of dietary PIPS soybean-derived phospholipid in obese Zucker (fa/fa) rats. Biosci. Biotechnol. Biochem. 2010, 74, 2333–2335. [Google Scholar] [CrossRef]
- Sprecher, H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochem. Biophys. Acta 2000, 1486, 219–231. [Google Scholar] [CrossRef]
- Pawar, A.; Jump, D.B. Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor α activity in rat primary hepatoctes. J. Biol. Chem. 2003, 278, 35931–35939. [Google Scholar] [CrossRef]
- Chakravarthy, M.V.; Lodhi, I.J.; Yin, L.; Malapaka, R.R.; Xu, H.E.; Turk, J.; Semenkovich, C.F. Identification of a physiologically relevant endogenous ligand for PPARα in liver. Cell 2009, 138, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Shimano, H.; Amemiya-Kudo, M.; Yahagi, N.; Hasty, A.H.; Matsuzaka, T.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol. Cell. Biol. 2001, 21, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Yahagi, N.; Matsuzaka, T.; Najima, Y.; Nakakuki, M.; Nagai, R.; Ishibashi, S.; Osuga, J.-I.; Yamada, N.; Shimano, H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 2003, 38, 1529–1539. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef]
- Hosokawa, M.; Shimatani, T.; Kanada, T.; Inoue, Y.; Takahashi, K. Conversion to docosahexaenoic acid-containing phosphatidylserine from squid skin lecithin by phospholipase D-mediated transphosphatidylation. J. Agric. Food Chem. 2000, 48, 4550–4554. [Google Scholar] [CrossRef]
- Lou, Q.M.; Wang, Y.M.; Liu, X.F.; Xue, C.H. Lipid profile and fatty acid compositions in body wall of Apostichopus japonicus (Selenka). J. Food Biochem. 2012, 36, 317–321. [Google Scholar] [CrossRef]





| g/100 g Body Weight | N | M | GPC | GPE | GPS | SPC | SPE |
|---|---|---|---|---|---|---|---|
| liver | 3.80 ± 0.08 | 3.02 ± 0.08 ab ** | 2.69 ± 0.14 a | 3.02 ± 0.14 ab | 3.15 ± 0.06 ab | 3.06 ± 0.12 ab | 3.01 ± 0.07 ab |
| kidney | 1.24 ± 0.03 | 1.10 ± 0.02 | 1.12 ± 0.04 | 1.14 ± 0.06 | 1.14 ± 0.04 | 1.17 ± 0.03 | 1.12 ± 0.04 |
| heart | 0.59 ± 0.03 | 0.50 ± 0.02 | 0.49 ± 0.03 | 0.48 ± 0.02 | 0.47 ± 0.02 | 0.50 ± 0.04 | 0.47 ± 0.03 |
| lung | 0.60 ± 0.01 | 0.47 ± 0.03 | 0.49 ± 0.06 | 0.44 ± 0.02 | 0.51 ± 0.03 | 0.50 ± 0.03 | 0.49 ± 0.03 |
| spleen | 0.22 ± 0.00 | 0.22 ± 0.02 | 0.23 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.23 ± 0.02 | 0.22 ± 0.01 |
| thymus | 0.18 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.00 | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 |
| muscle | 1.20 ± 0.01 | 0.96 ± 0.03 a ** | 1.00 ± 0.01 ab | 1.01 ± 0.04 abc | 1.06 ± 0.05 abc | 1.04 ± 0.03 abc | 1.00 ± 0.03 ab |
| g/100 g body weight | SPS | DPC | DPE | DPS | EPC | EPE | EPS |
| liver | 3.39 ± 0.14 bc | 3.10 ± 0.20 ab | 2.90 ± 0.07 ab | 3.16 ± 0.38 ab | 3.12 ± 0.12 ab | 3.35 ± 0.12 bc | 3.15 ± 0.15 ab |
| kidney | 1.21 ± 0.04 | 1.10 ± 0.07 | 1.12 ± 0.06 | 1.16 ± 0.15 | 1.15 ± 0.03 | 1.11 ± 0.01 | 1.24 ± 0.04 |
| heart | 0.58 ± 0.04 | 0.45 ± 0.04 | 0.48 ± 0.01 | 0.47 ± 0.06 | 0.51 ± 0.04 | 0.50 ± 0.03 | 0.55 ± 0.03 |
| lung | 0.56 ± 0.02 | 0.47 ± 0.03 | 0.45 ± 0.02 | 0.49 ± 0.06 | 0.48 ± 0.04 | 0.52 ± 0.02 | 0.53 ± 0.03 |
| spleen | 0.22 ± 0.02 | 0.27 ± 0.06 | 0.23 ± 0.02 | 0.23 ± 0.03 | 0.25 ± 0.02 | 0.23 ± 0.02 | 0.28 ± 0.02 |
| thymus | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.03 | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.17 ± 0.01 |
| muscle | 1.14 ± 0.03 bc | 0.99 ± 0.03 ab | 1.03 ± 0.06 abc | 1.05 ± 0.05 abc | 1.04 ± 0.03 abc | 1.09 ± 0.02 abc | 1.11 ± 0.02 bc |
| p-Value | FAs | Polar Head Groups | FAs × Polar Head Groups |
|---|---|---|---|
| Growth parameters | |||
| Body weight gain | NS | 0.04 | NS |
| vWAT | 0.01 | 0.025 | NS |
| Serum lipid profile | |||
| TG | NS | NS | NS |
| TC | <0.001 | 0.002 | NS |
| HDL-C | 0.002 | NS | NS |
| LDL-C | <0.001 | 0.002 | NS |
| Serum inflammation markers | |||
| TNF-α | <0.001 | <0.001 | NS |
| MCP-1 | <0.001 | 0.014 | <0.001 |
| Gene related to lipid oxidation | |||
| Cd36 | <0.001 | <0.001 | <0.001 |
| Cpt1a | NS | 0.006 | NS |
| Cpt2 | NS | <0.001 | NS |
| Acaa1a | <0.001 | <0.001 | 0.013 |
| Gene related to lipid synthesis | |||
| Srebf1 | <0.001 | <0.001 | 0.007 |
| Fas | <0.001 | <0.001 | NS |
| Scd1 | <0.001 | <0.001 | <0.001 |
| Acc | <0.001 | <0.001 | NS |
| Protein related to lipid synthesis | |||
| FAS | <0.001 | 0.001 | NS |
| ACC | <0.001 | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Mu, J.; Meng, J.; Su, W.; Li, J. Dietary Phospholipids Alleviate Diet-Induced Obesity in Mice: Which Fatty Acids and Which Polar Head. Mar. Drugs 2023, 21, 555. https://doi.org/10.3390/md21110555
Zhang L, Mu J, Meng J, Su W, Li J. Dietary Phospholipids Alleviate Diet-Induced Obesity in Mice: Which Fatty Acids and Which Polar Head. Marine Drugs. 2023; 21(11):555. https://doi.org/10.3390/md21110555
Chicago/Turabian StyleZhang, Lingyu, Jiaqin Mu, Jing Meng, Wenjin Su, and Jian Li. 2023. "Dietary Phospholipids Alleviate Diet-Induced Obesity in Mice: Which Fatty Acids and Which Polar Head" Marine Drugs 21, no. 11: 555. https://doi.org/10.3390/md21110555






