Biocompatibility of the Biopolymer Cyanoflan for Applications in Skin Wound Healing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cyanoflan Effect on Cell Viability or Apoptosis in Human Dermal Cell Lines
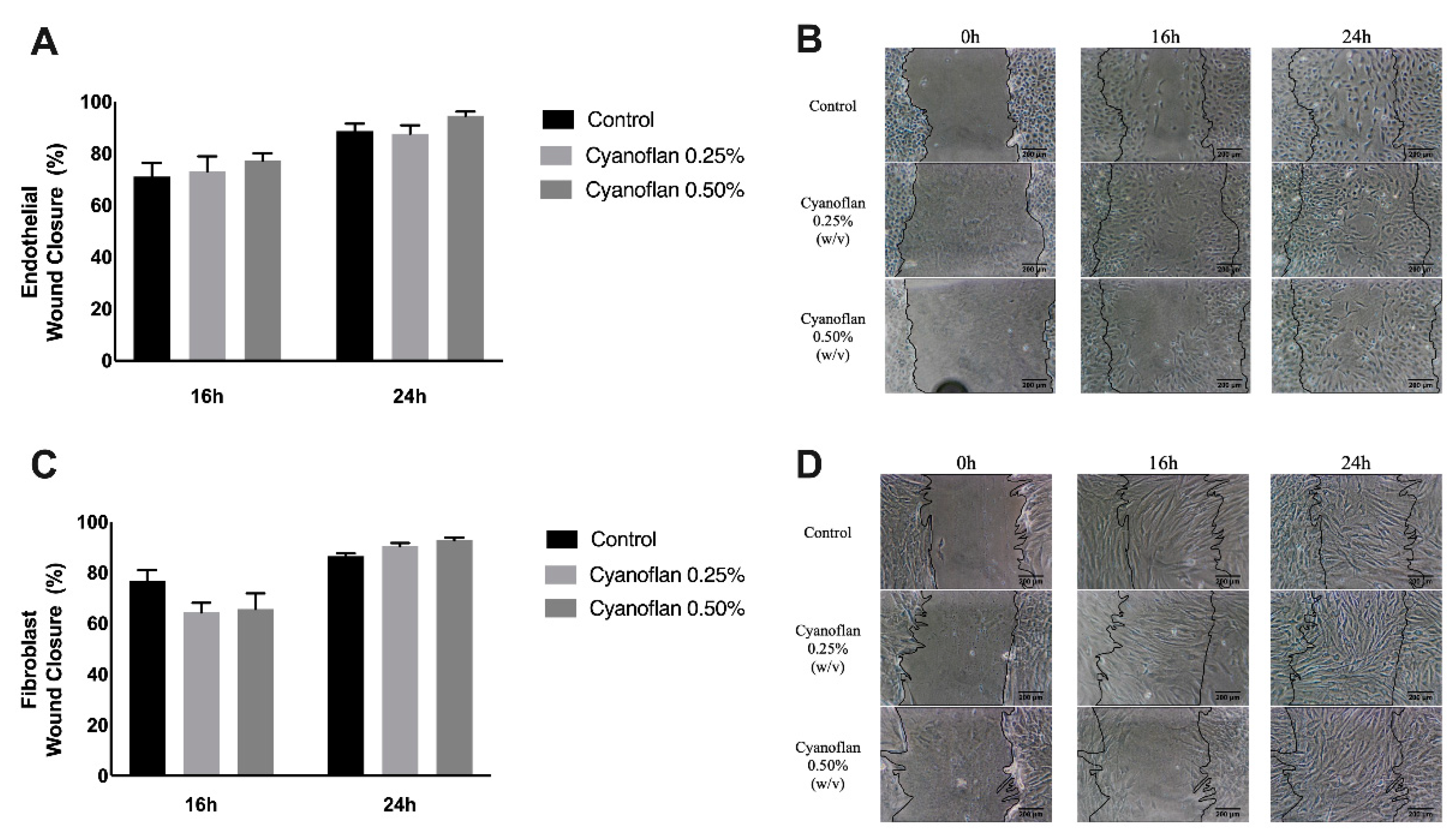
2.2. Cyanoflan Effect on Injury: In Vitro Assay
2.3. Reactive Oxygen Species (ROS) Production Assessment
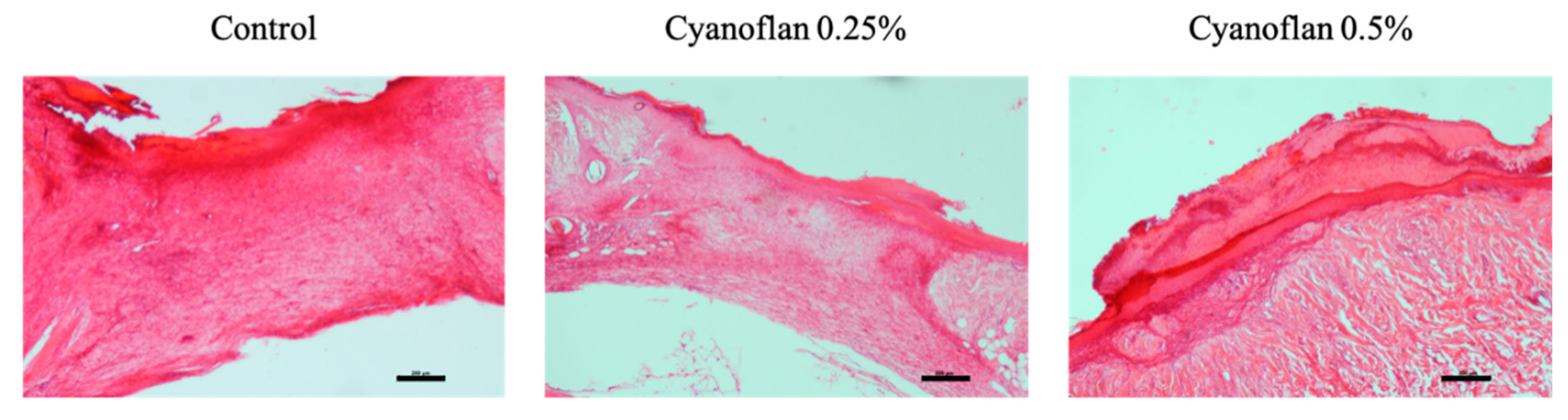
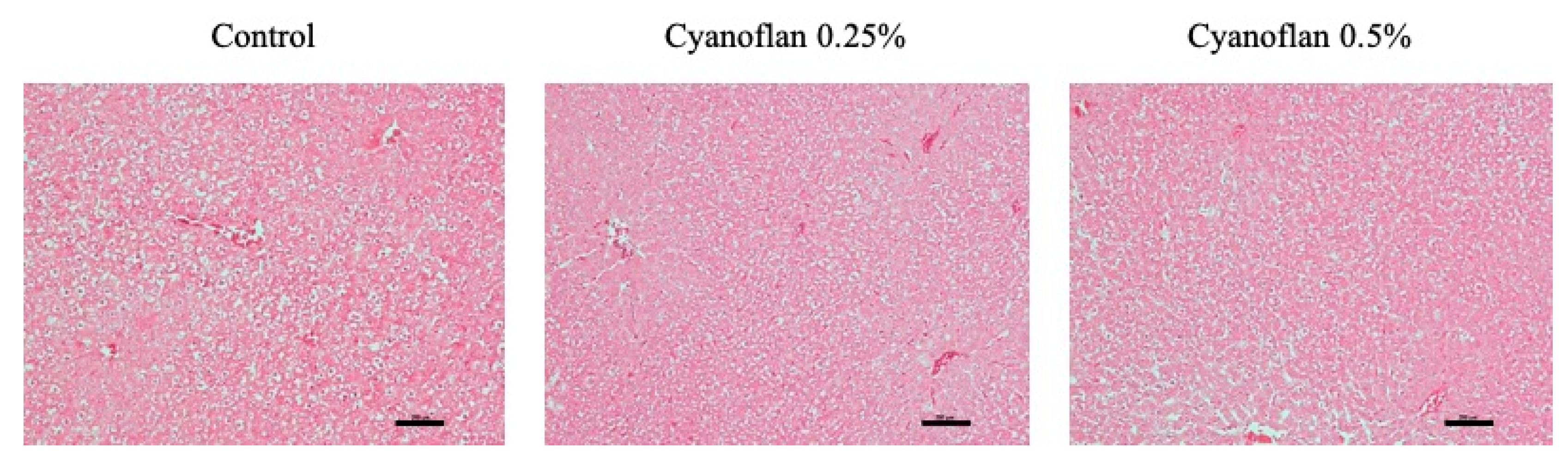
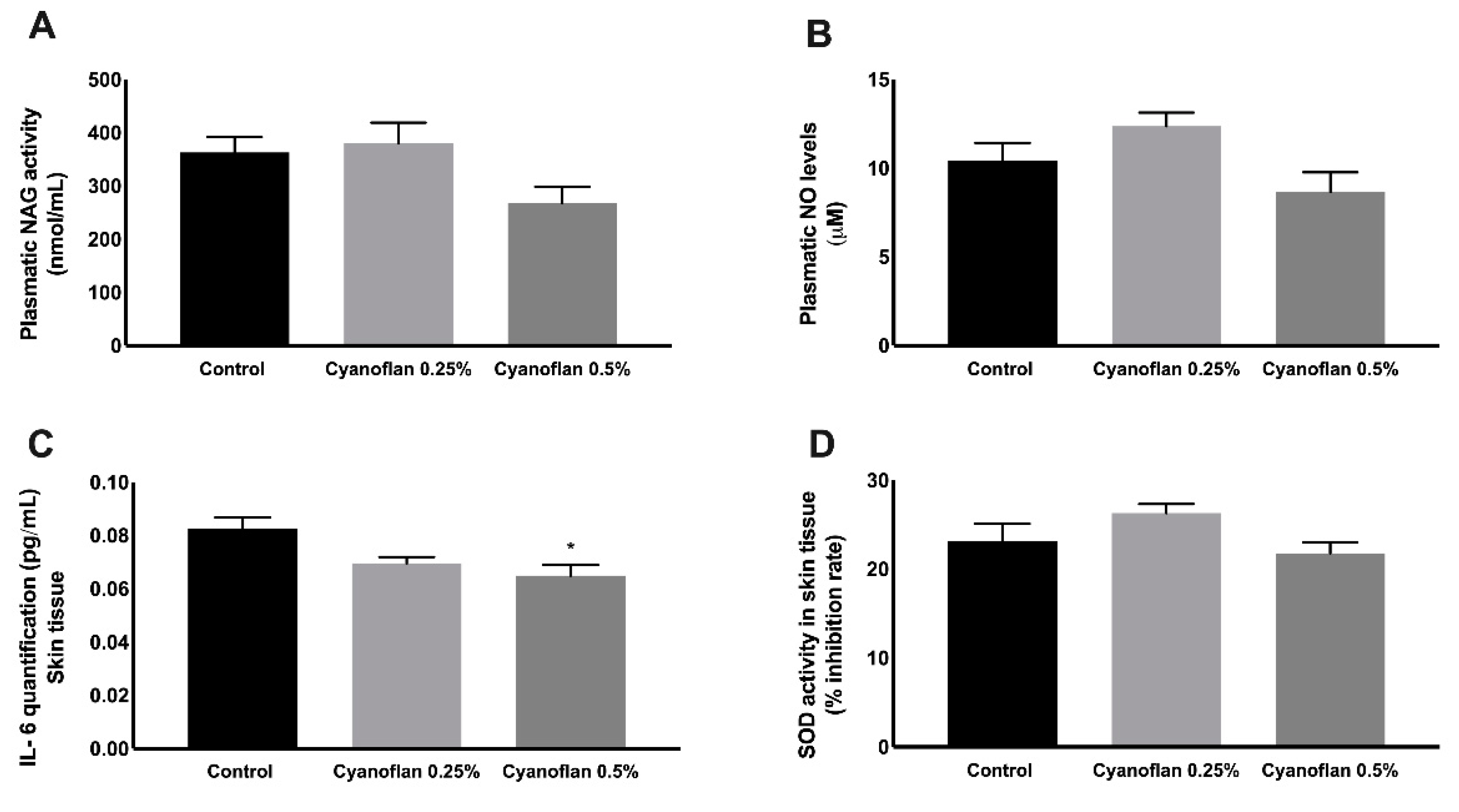
2.4. Bioactivity and Safety Application of Cyanoflan In Vivo
3. Materials and Methods
3.1. Cyanobacterium Growth Conditions and Cyanoflan Isolation
3.2. Cell Culture Experiments
3.3. Viability and Apoptosis Evaluation by Flow Cytometry
3.4. Migration Analysis by Injury Assay
3.5. Reactive Oxygen Species (ROS) Formation
3.6. In Vivo Biocompatibility Assay
3.7. Inflammatory Markers
3.7.1. Interleukin-6 Quantification
3.7.2. N-acetyl-ß-D-glucosaminidase (NAG) Determination Assay
3.7.3. Nitric Oxide (NO) Quantification
3.7.4. Superoxide Dismutase (SOD) Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair. Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, M.; Bhatnagar, A. Wound Dressings from Algal Polymers. In Marine Algae Extracts: Processes, Products, and Applications, 1st ed.; Kim, S.-K., Chojnacka, K., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2015; pp. 523–555. [Google Scholar]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Moxon, S.; Morris, G.A. Biopolymers as wound healing materials. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 261–287. [Google Scholar]
- Mota, R.; Vidal, R.; Pandeirada, C.; Flores, C.; Adessi, A.; De Philippis, R.; Nunes, C.; Coimbra, M.A.; Tamagnini, P. Cyanoflan: A cyanobacterial sulfated carbohydrate polymer with emulsifying properties. Carbohydr. Polym. 2020, 229, 115525. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, B.N.; Mota, R.; Leite, J.P.; Tamagnini, P.; Gales, L.; Rocha, F. Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12. Powder Technol. 2019, 343, 644–651. [Google Scholar] [CrossRef]
- Leite, J.P.; Mota, R.; Durao, J.; Neves, S.C.; Barrias, C.C.; Tamagnini, P.; Gales, L. Cyanobacterium-Derived Extracellular Carbohydrate Polymer for the Controlled Delivery of Functional Proteins. Macromol. Biosci. 2017, 17, 1600206. [Google Scholar] [CrossRef]
- Costa, B.; Mota, R.; Parreira, P.; Tamagnini, P.; MC, L.M.; Costa, F. Broad-Spectrum Anti-Adhesive Coating Based on an Extracellular Polymer from a Marine Cyanobacterium. Mar. Drugs 2019, 17, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, B.; Mota, R.; Tamagnini, P.; Martins, M.C.L.; Costa, F. Natural Cyanobacterial Polymer-Based Coating as a Preventive Strategy to Avoid Catheter-Associated Urinary Tract Infections. Mar. Drugs 2020, 18, 279. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing-A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [Green Version]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2014, 88, e51046. [Google Scholar]
- Mayol, L.; De Stefano, D.; Campani, V.; De Falco, F.; Ferrari, E.; Cencetti, C.; Matricardi, P.; Maiuri, L.; Carnuccio, R.; Gallo, A.; et al. Design and characterization of a chitosan physical gel promoting wound healing in mice. J. Mater. Sci. Mater. Med. 2014, 25, 1483–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, X.; Alves, A.; Ribeiro, M.P.; Lazzari, M.; Coutinho, P.; Otero, A. Biochemical characterization of Nostoc sp. exopolysaccharides and evaluation of potential use in wound healing. Carbohydr. Polym. 2021, 254, 117303. [Google Scholar] [CrossRef] [PubMed]
- Parwani, L.; Bhatnagar, M.; Bhatnagar, A.; Sharma, V. Antioxidant and iron-chelating activities of cyanobacterial exopolymers with potential for wound healing. J. Appl. Phycol. 2014, 26, 1473–1482. [Google Scholar] [CrossRef]
- Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Mota, R.; Rossi, F.; Andrenelli, L.; Pereira, S.B.; De Philippis, R.; Tamagnini, P. Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: Interactions between metals and RPS binding sites. Appl. Microbiol. Biotechnol. 2016, 100, 7765–7775. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Su, L.; Chen, S.; Zhao, L.; Wang, H.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical Characterization and Functional Analysis of the Polysaccharide from the Edible Microalga Nostoc sphaeroides. Molecules 2018, 23, 508. [Google Scholar] [CrossRef] [Green Version]
- Freeman, F.E.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, M.C.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, M.; Parwani, L.; Sharma, V.; Ganguly, J.; Bhatnagar, A. Exopolymers from Tolypothrix tenuis and three Anabaena sp (Cyanobacteriaceae) as novel blood clotting agents for wound management. Carbohydr. Polym. 2014, 99, 692–699. [Google Scholar] [CrossRef]
- Fukushima, S.; Motoyama, K.; Tanida, Y.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Tanaka, T.; Ihn, H.; Arima, H. Clinical evaluation of novel natural polysaccharides sacran as a skincare material for atopic dermatitis patients. J. Chem. Dermatol. Sci. Appl. 2016, 6, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.; Carneiro, A.; Rocha, A.; Pirraco, A.; Falcao, M.; Vasques, L.; Soares, R. Bevacizumab and Ranibizumab on Microvascular Endothelial Cells: A Comparative Study. J. Cell. Biochem. 2009, 108, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardao, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.; Negrao, R.; Valente, I.; Castela, A.; Duarte, D.; Guardao, L.; Magalhaes, P.J.; Rodrigues, J.A.; Guimaraes, J.T.; Gomes, P.; et al. Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J. Nat. Prod. 2013, 76, 2047–2053. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, R.; Costa, L.; Rodrigues, I.; Meireles, C.; Soares, R.; Tamagnini, P.; Mota, R. Biocompatibility of the Biopolymer Cyanoflan for Applications in Skin Wound Healing. Mar. Drugs 2021, 19, 147. https://doi.org/10.3390/md19030147
Costa R, Costa L, Rodrigues I, Meireles C, Soares R, Tamagnini P, Mota R. Biocompatibility of the Biopolymer Cyanoflan for Applications in Skin Wound Healing. Marine Drugs. 2021; 19(3):147. https://doi.org/10.3390/md19030147
Chicago/Turabian StyleCosta, Raquel, Luís Costa, Ilda Rodrigues, Catarina Meireles, Raquel Soares, Paula Tamagnini, and Rita Mota. 2021. "Biocompatibility of the Biopolymer Cyanoflan for Applications in Skin Wound Healing" Marine Drugs 19, no. 3: 147. https://doi.org/10.3390/md19030147






