Utilization of Seafood Processing By-Products for Production of Proteases by Paenibacillus sp. TKU052 and Their Application in Biopeptides’ Preparation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening, Selection, and Identification of Protease-Producing Bacterium
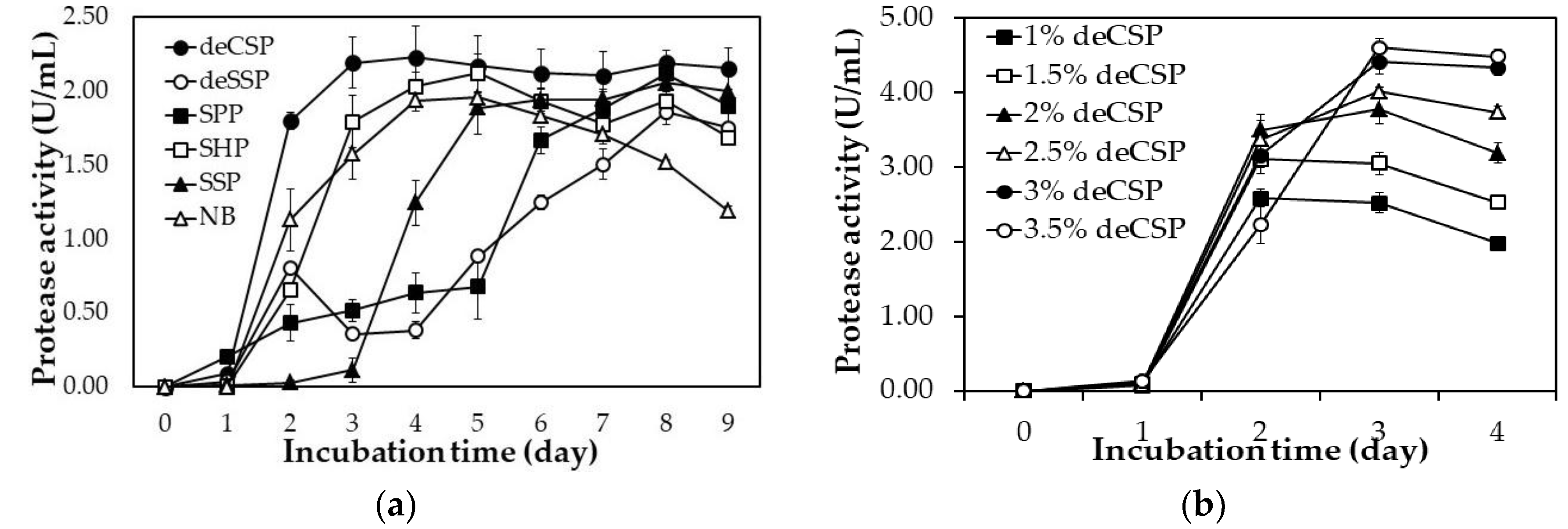
2.2. By-Products of Seafood Processing as the Sole C/N Source for Protease Production
2.3. Protease Purification
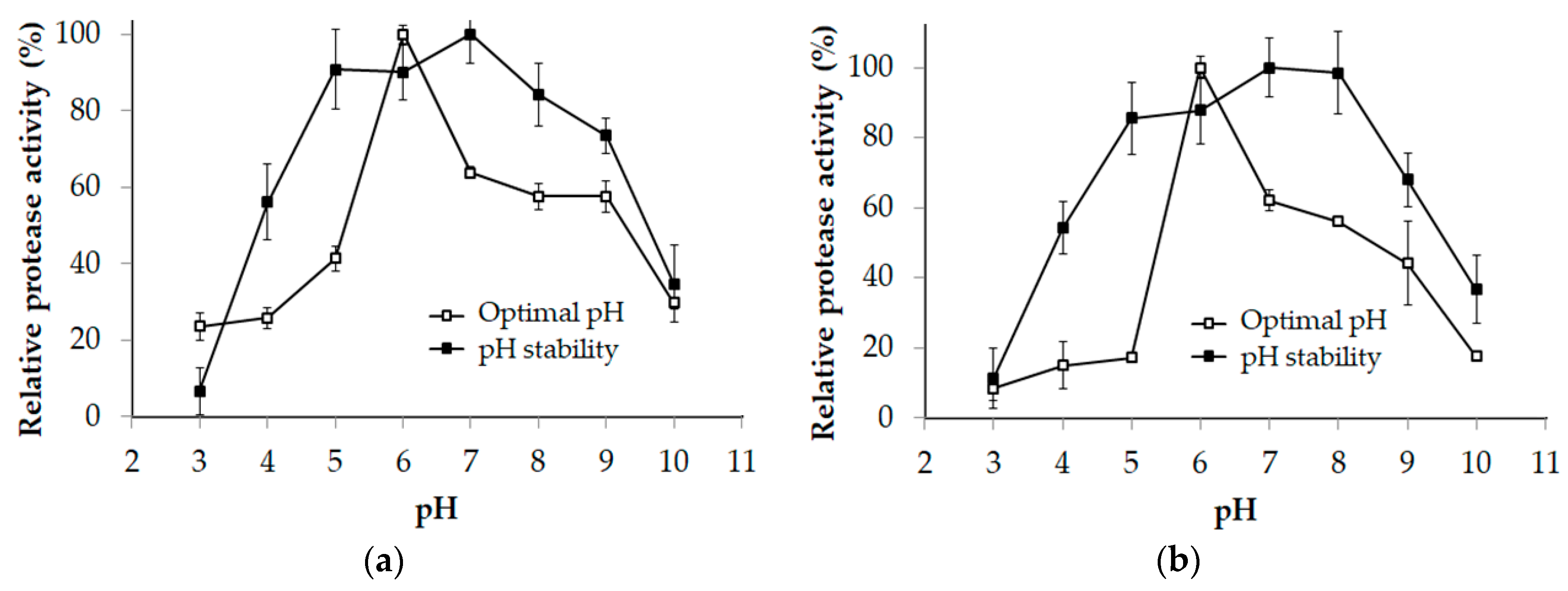
2.4. Effects of Temperature and pH on the Protease Activity and Stability
2.5. Effect of Metal Ion, Surfactants, and Protease Inhibitors
2.6. Substrate Specificity
2.7. Confirmation of Protease Production in Culture Medium
2.8. Gelatin Hydrolysis
2.9. Bioactivity Evaluation of Gelatin Hydrolysate
3. Materials and Methods
3.1. Materials
3.2. Screening, Selection, and Identification of the Protease-Producing Bacterium
3.3. Protease Assay
3.4. By-Products of Seafood Processing as the Sole C/N Source for Protease Production
3.5. Enzyme Purification
3.6. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
3.7. Zymography
3.8. Effect of Temperature and pH
3.9. Substrate Specificity
3.10. Effect of Metal Ions, Inhibitors, and Surfactants
3.11. Gelatin Hydrolysis
3.12. Evaluation of Gelatin Hydrolysate Bioactivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; kumar, V. A review on bioactive peptides: Physiological functions, bioavailability and safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.F.F.; Coimbra, J.S.R.; Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Bioactive peptides: Synthesis, properties, and applications in the packaging and preservation of food. Compr. Rev. Food Sci. Food Saf. 2012, 11, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Amigo, L.; Hernández-Ledesma, B. Current evidence on the bioavailability of food bioactive peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, F.; Wu, J. Perspectives on the potential benefits of antihypertensive peptides towards metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, M.; Abidin, N.B.Z.; Auwal, S.M.; Chay, S.Y.; Abdul Haiyee, Z.; Md Sikin, A.; Saari, N. Angiotensin converting enzyme (ACE)-peptide interactions: Inhibition kinetics, in silico molecular docking and stability study of three novel peptides generated from palm kernel cake proteins. Biomolecules 2019, 9, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [Green Version]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Halim, N.R.A.; Yosof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydrolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Wang, C.L.; Wang, S.L. Microbial conversion of shrimp heads to proteases and chitin as an effective dye adsorbent. Polymers 2020, 12, 2228. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Wen, I.H.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of shrimp head waste for production of a thermotolerant, detergent-stable, alkaline protease by Paenibacillus sp. Catalysts 2019, 9, 798. [Google Scholar] [CrossRef] [Green Version]
- Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Anti-α-glucosidase activity by a protease from Bacillus licheniformis. Molecules 2019, 24, 691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L. Microbial reclamation of squid pen. Biocatal. Agric. Biotechnol. 2012, 1, 177–180. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of squid pens to chitosanases and proteases via Paenibacillus sp. TKU042. Mar. Drugs 2018, 16, 83. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.L.; Chio, S.H. Deproteination of shrimp and crab shell with the protease of Pseudomonas aeruginosa K-187. Enzym. Microb. Technol. 1998, 22, 629–633. [Google Scholar] [CrossRef]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial proteases applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.M.; Kumar, R.; Panwar, S.; Kumar, A. Microbial alkaline proteases: Optimization of production parameters and their properties. J. Genet. Eng. Biotechnol. 2017, 15, 115–126. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, M.T.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. An exochitinase with N-acetyl-β-glucosaminidase-like activity from shrimp head conversion by Streptomyces speibonae and its application in hydrolyzing β-chitin powder to produce N-acetyl-D-glucosamine. Polymers 2019, 11, 1600. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019, 45, 727–742. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Production of a thermostable chitosanase from shrimp heads via Paenibacillus mucilaginosus TKU032 conversion and its application in the preparation of bioactive chitosan oligosaccharides. Mar. Drugs 2019, 17, 217. [Google Scholar] [CrossRef] [Green Version]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of marine chitinous materials for chitosanase production via microbial conversion by Paenibacillus macerans. Mar. Drugs 2018, 16, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Tran, T.D.; Nguyen, A.D.; Wang, S.L. Bioprocessing of squid pens waste into chitosanase by Paenibacillus sp. TKU047 and its application in low-molecular weight chitosan oligosaccharides production. Polymers 2020, 12, 1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Yu, H.T.; Tsai, M.H.; Doan, C.T.; Nguyen, V.B.; Do, V.C.; Nguyen, A.D. Conversion of squid pens to chitosanases and dye adsorbents via Bacillus cereus. Res. Chem. Intermed. 2018, 44, 4903–4911. [Google Scholar] [CrossRef]
- Wang, S.L.; Nguyen, V.B.; Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, A.D. Production and potential applications of bioconversion of chitin and protein-containing fishery byproducts into prodigiosin: A Review. Molecules 2020, 25, 2744. [Google Scholar] [CrossRef]
- Liang, T.W.; Wu, C.C.; Cheng, W.T.; Chen, Y.C.; Wang, C.L.; Wang, I.L.; Wang, S.L. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Appl. Biochem. Biotechnol. 2014, 172, 933–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.B.; Wang, S.L. Reclamation of marine chitinous materials for the production of α-glucosidase inhibitors via microbial conversion. Mar. Drugs 2017, 15, 350. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.B.; Wang, S.L. New novel α–glucosidase inhibitors produced by microbial conversion. Process Biochem. 2018, 65, 228–232. [Google Scholar] [CrossRef]
- Liang, T.W.; Tseng, S.C.; Wang, S.L. Production and characterization of antioxidant properties of exopolysaccharide(s) from Paenibacillus mucilaginosus TKU032. Mar. Drugs 2016, 14, 40. [Google Scholar] [CrossRef]
- Wang, S.L.; Liang, T.W. Microbial reclamation of squid pens and shrimp shell. Res. Chem. Intermed. 2017, 43, 3445–3462. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, H.P.; Sonawane, M.S.; Khairnar, M.H.; Sayyed, R.Z. Production of alkaline protease by rhizospheric Bacillus cereus HP_RZ17 and Paenibacillus xylanilyticus HP_RZ19. Environ. Sustain. 2020, 3, 5–13. [Google Scholar] [CrossRef]
- Girardin, H.; Albagnac, C.; Dargaignaratz, C.; Nguyen-The, C.; Carlin, F. Antimicrobial activity of foodborne Paenibacillus and Bacillus spp. against Clostridium botulinum. J. Food Prot. 2002, 65, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.-W.; Wang, S.-L. Recent Advances in Exopolysaccharides from Paenibacillus spp.: Production, Isolation, Structure, and Bioactivities. Mar. Drugs 2015, 13, 1847–1863. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Leila, R.; Zahra, R.; Pegah, M.; Mozafar, K. Antiproliferatory effects of crab shell extract on breast cancer cell line (MCF7). J. Breast Cancer 2014, 17, 219–225. [Google Scholar]
- Soundarapandian, P.; Shyamalendu, R.; Varadharajan, D. Antioxidant activity in hard and soft shell crabs of Charybdis lucifera (Fabricius, 1798). J. Aquac. Res. Dev. 2014, 5, 7. [Google Scholar]
- Rai, S.K.; Roy, J.K.; Mukherjee, A.K. Characterisation of a detergent-stable alkaline protease from a novel thermophilic strain Paenibacillus tezpurensis sp. nov. AS-S24-II. Appl. Microbiol. Biotechnol. 2009, 85, 1437–1450. [Google Scholar] [CrossRef]
- Alvarez, V.M.; von der Weid, I.; Seldin, L.; Santos, A.L.S. Influence of growth conditions on the production of extracellular proteolytic enzymes in Paenibacillus peoriae NRRL BD-62 and Paenibacillus polymyxa SCE2. Lett. Appl. Microbiol. 2006, 43, 625–630. [Google Scholar] [CrossRef]
- Antúnez, K.; Arredondo, D.; Anido, M.; Zunino, P. Metalloprotease production by Paenibacillus larvae during the infection of honeybee larvae. Microbiology 2011, 157, 1474–1480. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Pan, Y.; She, Q.; Chen, L. A novel carboxyl-terminal protease derived from Paenibacillus lautus CHN26 exhibiting high activities at multiple sites of substrates. BMC Biotechnol. 2013, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Paul, T.; Das, A.; Mandal, A.; Jana, A.; Maity, C.; Adak, A.; Halder, S.K.; DasMohapatra, P.K.; Pati, B.R.; Mondal, K.C. Effective dehairing properties of keratinase from Paenibacillus woosongensis TKB2 obtained under solid state fermentation. Waste Biomass Valoriz. 2014, 5, 97–107. [Google Scholar] [CrossRef]
- Mahmoodani, F.; Ghassem, M.; Babji, A.S.; Yusop, S.M.; Khosrokhavar, R. ACE inhibitory activity of pangasius catfish (Pangasius sutchi) skin and bone gelatin hydrolysate. J. Food Sci. Technol. 2014, 51, 1847–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibitory activity, antioxidant properties, phenolic content and amino acid profiles of Fucus spiralis L. Protein hydrolysate fractions. Mar. Drugs 2017, 15, 311. [Google Scholar] [CrossRef]
- Andrews, P.R.; Carson, J.M.; Caselli, A.; Spark, M.J.; Woods, R. Conformational analysis and active site modelling of angiotensin-converting enzyme inhibitors. J. Med. Chem. 1985, 28, 393–399. [Google Scholar] [CrossRef]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Li, H.; Liu, X. The potential of proteins, hydrolysates and peptides as growth factors for Lactobacillus and Bifidobacterium: Current research and future perspectives. Food Funct. 2020, 11, 1946–1957. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature 1970, 257, 680–685. [Google Scholar] [CrossRef]
- Morais, H.A.; Silvestre, M.P.C.; Silva, V.D.M.; Silva, M.R.; Silva, A.C.S.; Silveira, J.N. Correlation between the degree of hydrolysis and the peptide profile of whey protein concentrate hydrolysates: Effect of the enzyme type and reaction time. Am. J. Food Technol. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Anti-oxidant and anti-diabetes potential of water-soluble chitosan–glucose derivatives produced by Maillard reaction. Polymers 2019, 11, 1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]








| Step | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|
| Cultural supernatant | 1557.35 | 162.5 | 0.10 | 100.00 | 1.00 |
| (NH4)2SO4 precipitation | 174.21 | 148.2 | 0.85 | 91.20 | 8.15 |
| Ion-exchange chromatography | |||||
| P1 | 2.16 | 24.91 | 11.52 | 15.33 | 110.41 |
| P2 | 1.83 | 56.23 | 30.74 | 34.60 | 294.61 |
| Gel filtration | |||||
| P1 | 0.59 | 9.42 | 15.84 | 5.80 | 151.83 |
| P2 | 0.87 | 43.07 | 49.44 | 26.50 | 473.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Utilization of Seafood Processing By-Products for Production of Proteases by Paenibacillus sp. TKU052 and Their Application in Biopeptides’ Preparation. Mar. Drugs 2020, 18, 574. https://doi.org/10.3390/md18110574
Doan CT, Tran TN, Nguyen VB, Nguyen AD, Wang S-L. Utilization of Seafood Processing By-Products for Production of Proteases by Paenibacillus sp. TKU052 and Their Application in Biopeptides’ Preparation. Marine Drugs. 2020; 18(11):574. https://doi.org/10.3390/md18110574
Chicago/Turabian StyleDoan, Chien Thang, Thi Ngoc Tran, Van Bon Nguyen, Anh Dzung Nguyen, and San-Lang Wang. 2020. "Utilization of Seafood Processing By-Products for Production of Proteases by Paenibacillus sp. TKU052 and Their Application in Biopeptides’ Preparation" Marine Drugs 18, no. 11: 574. https://doi.org/10.3390/md18110574









