Development of Injectable Fucoidan and Biological Macromolecules Hybrid Hydrogels for Intra-Articular Delivery of Platelet-Rich Plasma
Abstract
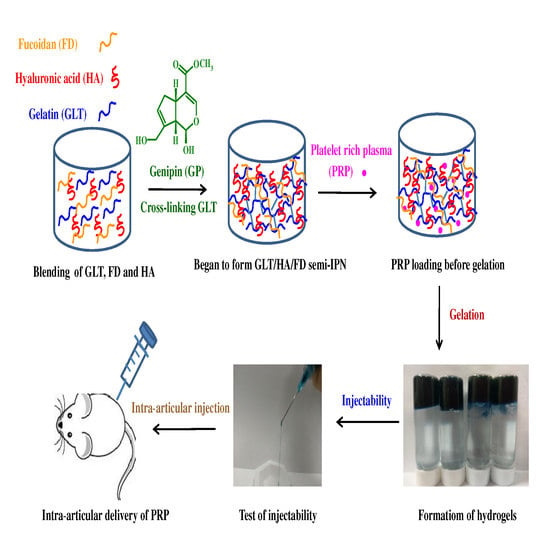
:1. Introduction
2. Results and Discussion
2.1. Depolymerization of Fucoidan
2.2. Cross-Linking Reaction and Gel Formation
2.3. Injectability, Stability and Adhesive Property
2.4. Rheological Property, Compressive Strength and Enzymatic Degradation
2.5. Cytotoxicity and Growth Factor Release
2.6. In Vivo Histological Evaluation
3. Materials and Methods
3.1. Materials
3.2. Preparation of LMWF
3.3. Characterization of Anti-Inflammatory and ROS Scavenging Effects
3.3.1. Colorimetric Nitric Oxide Assay
3.3.2. Enzyme-Linked Immunosorbent Assay (ELISA) for IL-6
3.3.3. Analysis of ROS Generation
3.4. Preparation of Injectable Hydrogels
3.5. UV-Vis Absorption Spectra
3.6. Fourier Transform Infrared Spectroscopy (FTIR)
3.7. Rheological Characterization
3.8. Gelation Time Determination
3.9. Compressive Mechanical Properties
3.10. Cytotoxicity
3.11. In Vitro Biodegradation Study
3.12. PRP-Loading and Release
3.13. Animal Model and Design
3.14. Specimen Collection and Histological (Microscopic) Examination
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.R.; Park, K.M.; Joung, Y.K.; Park, K.D.; Do, S.H. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J. Control. Release 2012, 159, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Yang, Y.L.; Niu, X.; Lin, Q.N.; Zhao, B.Z.; Wang, Y.; Zhu, L.Y. An in situ photocrosslinkable platelet rich plasma—Complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 2017, 62, 179–187. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y.F. Autologous liquid platelet rich fibrin: A novel drug delivery system. Acta Biomater. 2018, 75, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Jooybar, E.; Abdekhodaie, M.J.; Alvi, M.; Mousavi, A.; Karperien, M.; Dijkstra, P.J. An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomater. 2019, 83, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Anastyuk, S.D.; Ishina, I.A.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Zadorozhny, P.A.; Dmitrenok, P.S.; Ermakova, S.P. Structural characteristics and anticancer activity in vitro of fucoidan from brown alga Padina boryana. Carbohydr. Polym. 2018, 184, 260–268. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Lee, J.S.; Kim, W.S.; Jeon, Y.J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef]
- Bouvard, C.; Galy-Fauroux, I.; Grelac, F.; Carpentier, W.; Lokajczyk, A.; Gandrille, S.; Colliec-Jouault, S.; Fischer, A.M.; Helley, D. Low-molecular-weight fucoidan induces endothelial cell migration via the PI3K/AKT pathway and modulates the transcription of genes involved in angiogenesis. Mar. Drugs 2015, 13, 7446–7462. [Google Scholar] [CrossRef]
- Wang, F.L.; Schmidt, H.; Pavleska, D.; Wermann, T.; Seekamp, A.; Fuchs, S. Crude fucoidan extracts impair angiogenesis in models relevant for bone regeneration and osteosarcoma via reduction of VEGF and SDF-1. Mar. Drugs 2017, 15, 186. [Google Scholar] [CrossRef]
- Wu, S.J.; Don, T.M.; Lin, C.W.; Mi, F.L. Delivery of berberine using chitosan/fucoidan-taurine conjugate nanoparticles for treatment of defective intestinal epithelial tight junction barrier. Mar. Drugs 2014, 12, 5677–5697. [Google Scholar] [CrossRef]
- Yu, H.H.; Ko, E.C.; Chang, C.L.; Yuan, K.S.P.; Wu, A.T.H.; Shan, Y.S.; Wu, S.Y. Fucoidan inhibits radiation-induced pneumonitis and lung fibrosis by reducing inflammatory cytokine expression in lung tissues. Mar. Drugs 2018, 16, 392. [Google Scholar] [CrossRef] [PubMed]
- Subash, A.; Veeraraghavan, G.; Sali, V.K.; Bhardwaj, M.; Vasanthi, H.R. Attenuation of inflammation by marine algae Turbinaria ornata in cotton pellet induced granuloma mediated by fucoidan like sulphated polysaccharide. Carbohydr. Polym. 2016, 151, 1261–1268. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.; Kim, J.S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W.B. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Mourao, P.A.S. Perspective on the use of sulfated polysaccharides from marine organisms as a source of new antithrombotic drugs. Mar. Drugs 2015, 13, 2770–2784. [Google Scholar] [CrossRef]
- Jin, W.H.; Zhang, Q.B.; Wang, J.; Zhang, W.J. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Phull, A.R.; Majid, M.; Haq, I.U.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef]
- Jun, J.Y.; Jung, M.J.; Jeong, I.H.; Yamazaki, K.; Kawai, Y.; Kim, B.M. Antimicrobial and antibiofilm activities of sulfated polysaccharides from marine algae against dental plaque bacteria. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.X.; Cao, M.J.; Liu, G.M.; Chen, Q.C.; Sun, L.C.; Chen, H.X. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- Carson, M.A.; Clarke, S.A. Bioactive compounds from marine organisms: Potential for bone growth and healing. Mar. Drugs 2018, 16, 340. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Yan, M.D.; Lin, H.T.; Li, K.L.; Lin, Y.C. Toxicological evaluation of low molecular weight fucoidan in vitro and in vivo. Mar. Drugs 2016, 14, 121. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.N.; Letourneur, D.; Chaubet, F. Fucoidans in nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.C.; Chen, C.H.; Lin, C.W.; Ho, Y.C.; Mi, F.L. Development of mutlifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. Int. J. Biol. Macromol. 2019, 126, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lin, Y.S.; Wu, S.J.; Mi, F.L. Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym. 2018, 193, 163–172. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lu, K.Y.; Tseng, C.L.; Wu, J.Y.; Chen, C.H.; Mi, F.L. Development of genipin-crosslinked fucoidan/chitosan-N-arginine nanogels for preventing Helicobacter infection. Nanomedicine 2017, 12, 1491–1510. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.Y.; Li, R.; Hsu, C.H.; Lin, C.W.; Chou, S.C.; Tsai, M.L.; Mi, F.L. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydr. Polym. 2017, 165, 410–420. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.T.; Lu, T.W.; Chen, C.H.; Mi, F.L. Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 128, 973–984. [Google Scholar] [CrossRef]
- Lu, H.T.; Lu, T.W.; Chen, C.H.; Lu, K.Y.; Mi, F.L. Development of nanocomposite scaffolds based on biomineralization of N,O-carboxymethyl chitosan/fucoidan conjugates for bone tissue engineering. Int. J. Biol. Macromol. 2018, 120, 2335–2345. [Google Scholar] [CrossRef]
- Sumayya, A.S.; Muraleedhara Kurup, G. Biocompatibility of subcutaneously implanted marine macromolecules cross-linked bio-composite scaffold for cartilage tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, P.; Murali, M.R.; Samuel, S.; Raghavendran, H.R.B.; Abbas, A.A.; Kamarul, T. Three dimensional alginate-fucoidan composite hydrogel augments the chondrogenic differentiation of mesenchymal stromal cells. Carbohydr. Polym. 2016, 147, 294–303. [Google Scholar] [CrossRef]
- Sudirman, S.; Ong, A.D.; Chang, H.W.; Kong, Z.L. Effect of fucoidan on anterior cruciate ligament transection and medial meniscectomy induced osteoarthritis in high-fat diet-induced obese rats. Nutrients 2018, 10, 686. [Google Scholar] [CrossRef]
- Park, S.B.; Chun, K.R.; Kim, J.K.; Suk, K.; Jung, Y.M.; Lee, W.H. The differential effect of high and low molecular weight fucoidans on the severity of collagen-induced arthritis in mice. Phytother. Res. 2010, 24, 1384–1391. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, S.Y.; Chung, W.S.; Park, J.H.; Hwang, E.; Mavlonov, G.T.; Kim, I.H.; Kim, K.Y.; Yi, T.H. Fucoidan prevents the progression of osteoarthritis in rats. J. Med. Food 2015, 18, 1032–1041. [Google Scholar] [CrossRef]
- Myers, S.P.; Mulder, A.M.; Baker, D.G.; Robinson, S.R.; Rolfe, M.I.; Brooks, L.; Fitton, J.H. Effects of fucoidan from Fucus vesiculosus in reducing symptoms of osteoarthritis: A randomized placebo-controlled trial. Biologics 2016, 10, 81–88. [Google Scholar] [PubMed]
- Park, S.J.; Lee, K.W.; Lim, D.S.; Lee, S. The sulfated polysaccharide fucoidan stimulates osteogenic differentiation of human adipose-derived stem cells. Stem Cells Dev. 2012, 21, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Manzo-Silberman, S.; Louedec, L.; Meilhac, O.; Letourneur, D.; Michel, J.B.; Elmadbouh, I. Therapeutic potential of fucoidan in myocardial ischemia. J. Cardiovasc. Pharmacol. 2011, 58, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Liu, T.J. Mobilization of mesenchymal stem cells by stromal cell-derived factor-1 released from chitosan/tripolyphosphate/fucoidan nanoparticles. Acta Biomater. 2012, 8, 1048–1056. [Google Scholar] [CrossRef]
- Hamidi, S.; Letourneur, D.; Aid-Launais, R.; Di Stefano, A.; Vainchenker, W.; Norol, F.; Le Visage, C. Fucoidan promotes early step of cardiac differentiation from human embryonic stem cells and long-term maintenance of beating areas. Tissue Eng. Part A 2014, 20, 1285–1294. [Google Scholar] [CrossRef]
- Purnama, A.; Aid-Launais, R.; Haddad, O.; Maire, M.; Mantovani, D.; Letourneur, D.; Hlawaty, H.; Le Visage, C. Fucoidan in a 3D scaffold interacts with vascular endothelial growth factor and promotes neovascularization in mice. Drug Deliv. Transl. Res. 2015, 5, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.Y.; Huang, Y.C. Basic fibroblast growth factor released from fucoidan-modified chitosan/alginate scaffolds for promoting fibroblasts migration. J. Polym. Res. 2018, 25, 83. [Google Scholar] [CrossRef]
- Rujitanaroj, P.O.; Aid-Launais, R.; Chew, S.Y.; Le Visage, C. Polysaccharide electrospun fibers with sulfated poly(fucose) promote endothelial cell migration and VEGF-mediated angiogenesis. Biomater. Sci. 2014, 2, 843–852. [Google Scholar] [CrossRef]
- Marinval, N.; Morenc, M.; Labour, M.N.; Samotus, A.; Mzyk, A.; Ollivier, V.; Maire, M.; Jesse, K.; Bassand, K.; Niemiec-Cyganek, A.; et al. Fucoidan/VEGF-based surface modification of decellularized pulmonary heart valve improves the antithrombotic and re-endothelialization potential of bioprostheses. Biomaterials 2018, 172, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Yang, Y.T. Effect of basic fibroblast growth factor released from chitosan-fucoidan nanoparticles on neurite extension. J. Tissue Eng. Regen. Med. 2016, 10, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Wu, S.J.; Wu, J.Y.; Wen, D.Y.; Mi, F.L. Preparation of fucoidan-shelled and genipin-crosslinked chitosan beads for antibacterial application. Carbohydr. Polym. 2015, 126, 97–107. [Google Scholar] [CrossRef]
- Yu, S.H.; Wu, S.J.; Tang, D.W.; Ho, Y.C.; Mi, F.L.; Kuo, T.H.; Sung, H.W. Stimuli-responsive materials prepared from carboxymethyl chitosan and poly(gamma-glutamic acid) for protein delivery. Carbohydr. Polym. 2012, 87, 531–536. [Google Scholar] [CrossRef]
- Mi, F.L. Synthesis and characterization of a novel chitosan-gelatin bioconjugate with fluorescence emission. Biomacromolecules 2005, 6, 975–987. [Google Scholar] [CrossRef]
- Mi, F.L.; Tan, Y.C.; Liang, H.F.; Sung, H.W. In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 2002, 23, 181–191. [Google Scholar] [CrossRef]
- Mi, F.L.; Sung, H.W.; Shyu, S.S. Synthesis and characterization of a novel chitosan-based network prepared using naturally occurring crosslinker. J. Polym. Sci. Pol. Chem. 2000, 38, 2804–2814. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Cifuentes, A.; Rodriguez, G.; Aguilar, M.R.; Gonzalez-Gomez, A.; Solis, R.; Garcia-Honduvilla, N.; Bujan, J.; Garcia-Sanmartin, J.; Martinez, A.; et al. Bioactive bilayered dressing for compromised epidermal tissue regeneration with sequential activity of complementary agents. Acta Biomater. 2015, 23, 103–115. [Google Scholar] [CrossRef]
- Yang, W.N.; Chen, P.W.; Huang, C.Y. Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of crude extracts of fucoidan prepared from compressional puffing-pretreated sargassum crassifolium. Mar. Drugs 2017, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.V.; Tsou, Y.C.; Chen, Y.T.; Lu, W.J.; Hwang, P.A. Effects of low-molecular-weight fucoidan and high stability fucoxanthin on glucose homeostasis, lipid metabolism, and liver function in a mouse model of type ii diabetes. Mar. Drugs 2017, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.M.; Li, K.L.; Lin, Y.C. Fucoidan-fucoxanthin ameliorated cardiac function via IRS1/GRB2/ SOS1, GSK3 beta/CREB pathways and metabolic pathways in senescent mice. Mar. Drugs 2019, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Zhu, W.L.; Wang, T.T.; Jin, L.Y.; Liu, T.W.; Li, X.; Guan, Z.J.; Jiang, Z.F.; Meng, X.Z.; Wang, J.G.; et al. Low molecule weight fucoidan mitigates atherosclerosis in ApoE (-/-) mouse model through activating multiple signal pathway. Carbohydr. Polym. 2019, 206, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Xu, J.; Ge, K.L.; Tian, Q.W.; Zhao, P.; Guo, Y.L. Anti-inflammatory effect of low molecular weight fucoidan from Saccharina japonica on atherosclerosis in apoE-knockout mice. Int. J. Biol. Macromol. 2018, 118, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Nomura, K.; Nagashima, M.; Kamimura, N. Fucoidan alleviates high-fat diet-induced dyslipidemia and atherosclerosis in ApoE(Shl) mice deficient in apolipoprotein E expression. J. Nutr. Biochem. 2016, 32, 46–54. [Google Scholar] [CrossRef]
- Moraes, J.A.; Frony, A.C.; Barcellos-de-Souza, P.; Menezes da Cunha, M.; Brasil Barbosa Calcia, T.; Benjamim, C.F.; Boisson-Vidal, C.; Barja-Fidalgo, C. Downregulation of microparticle release and pro-inflammatory properties of activated human polymorphonuclear neutrophils by LMW fucoidan. J. Innate. Immun. 2018, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Yoon, K.Y.; Lee, B.Y. Low molecular weight fucoidan from the sporophyll of Undaria pinnatifida suppresses inflammation by promoting the inhibition of mitogen-activated protein kinases and oxidative stress in RAW 264.7 cells. Fitoterapia 2012, 83, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. BBA Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Pol. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Liang, H.C.; Chang, W.H.; Liang, H.F.; Lee, M.H.; Sung, H.W. Crosslinking structures of gelatin hydrogels crosslinked with genipin or a water-soluble carbodiimide. J. Appl. Polym. Sci. 2004, 91, 4017–4026. [Google Scholar] [CrossRef]
- Luo, J.W.; Liu, C.; Wu, J.H.; Lin, L.X.; Fan, H.M.; Zhao, D.H.; Zhuang, Y.Q.; Sun, Y.L. In situ injectable hyaluronic acid/gelatin hydrogel for hemorrhage control. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Ge, J.; Guo, B.L.; Ma, P.X. In situ forming biodegradable electroactive hydrogels. Polym. Chem. 2014, 5, 2880–2890. [Google Scholar] [CrossRef]
- Shankar, K.G.; Gostynska, N.; Montesi, M.; Panseri, S.; Sprio, S.; Kon, E.; Marcacci, M.; Tampieri, A.; Sandri, M. Investigation of different cross-linking approaches on 3D gelatin scaffolds for tissue engineering application: A comparative analysis. Int. J. Biol. Macromol. 2017, 95, 1199–1209. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, J.J.; Atala, A.; Lee, S.J. The effect of controlled release of PDGF-BB from heparin-conjugated electrospun PCL/gelatin scaffolds on cellular bioactivity and infiltration. Biomaterials 2012, 33, 6709–6720. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.T.; Sheu, M.T.; Lin, Y.F.; Lan, J.; Chin, Y.P.; Hsieh, M.S.; Cheng, C.W.; Chen, C.H. Injectable hyaluronic-acid-doxycycline hydrogel therapy in experimental rabbit osteoarthritis. BMC Vet. Res. 2013, 9, 68. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.-T.; Chang, W.-T.; Tsai, M.-L.; Chen, C.-H.; Chen, W.-Y.; Mi, F.-L. Development of Injectable Fucoidan and Biological Macromolecules Hybrid Hydrogels for Intra-Articular Delivery of Platelet-Rich Plasma. Mar. Drugs 2019, 17, 236. https://doi.org/10.3390/md17040236
Lu H-T, Chang W-T, Tsai M-L, Chen C-H, Chen W-Y, Mi F-L. Development of Injectable Fucoidan and Biological Macromolecules Hybrid Hydrogels for Intra-Articular Delivery of Platelet-Rich Plasma. Marine Drugs. 2019; 17(4):236. https://doi.org/10.3390/md17040236
Chicago/Turabian StyleLu, Hsien-Tsung, Wan-Ting Chang, Min-Lang Tsai, Chien-Ho Chen, Wei-Yu Chen, and Fwu-Long Mi. 2019. "Development of Injectable Fucoidan and Biological Macromolecules Hybrid Hydrogels for Intra-Articular Delivery of Platelet-Rich Plasma" Marine Drugs 17, no. 4: 236. https://doi.org/10.3390/md17040236