Raistrickiones A−E from a Highly Productive Strain of Penicillium raistrickii Generated through Thermo Change
Abstract
:1. Introduction
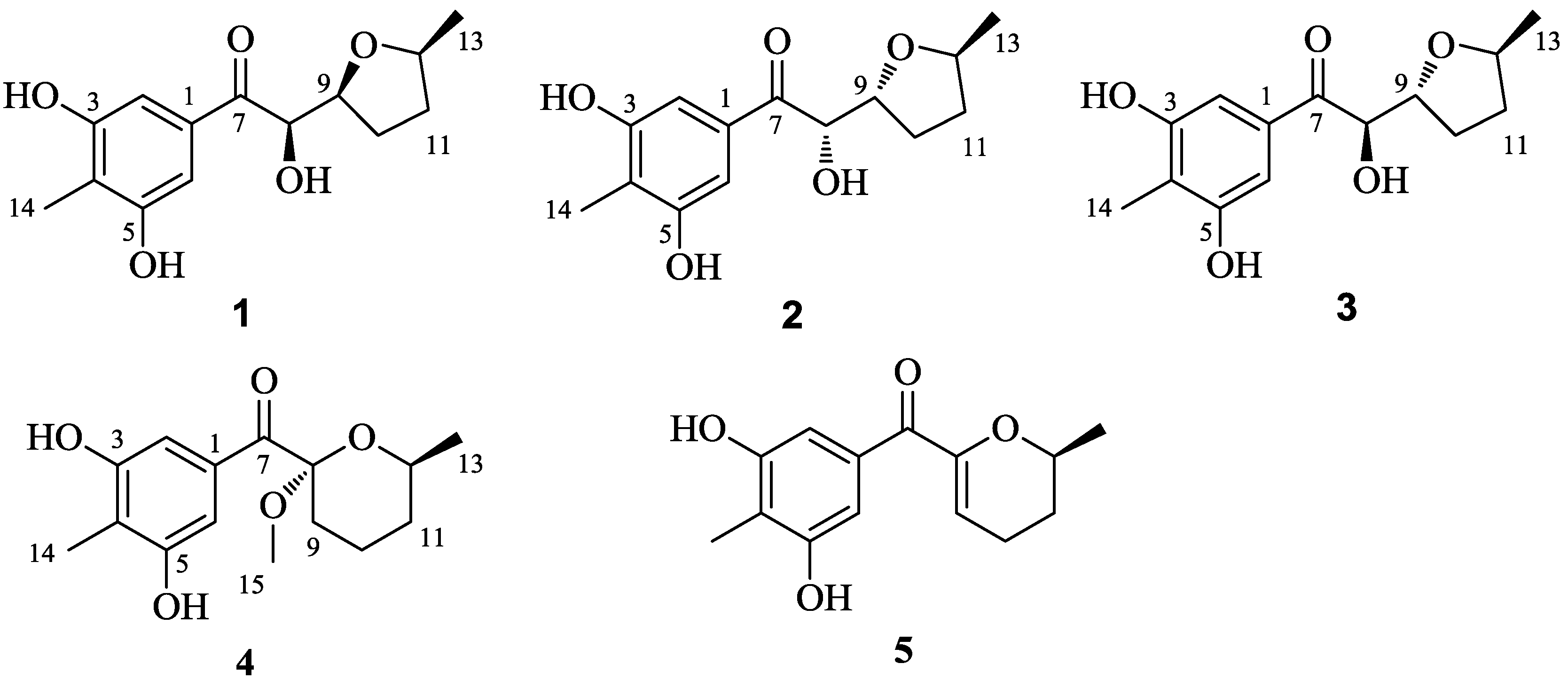
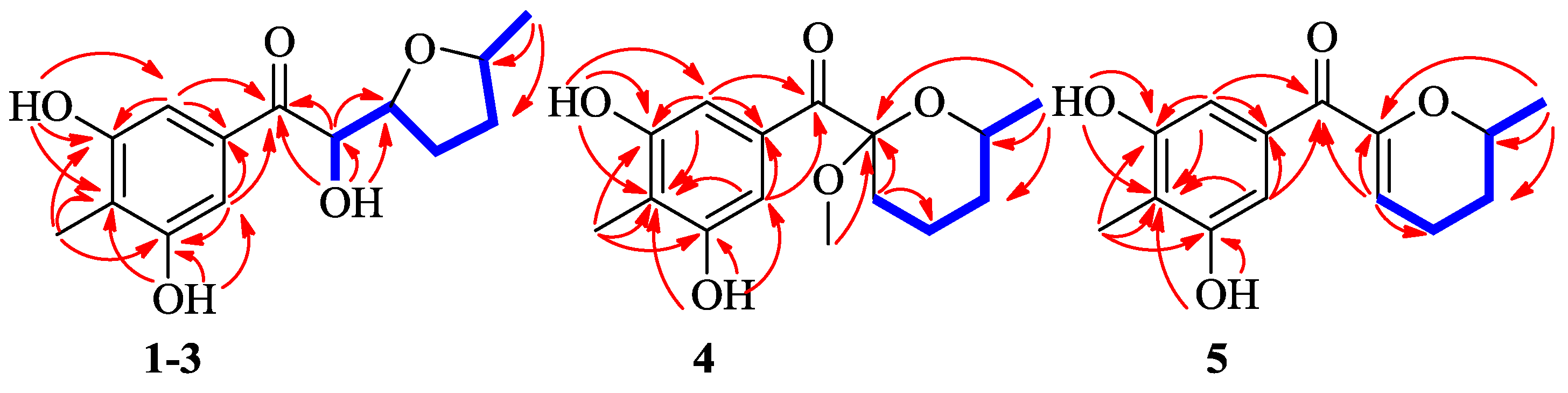
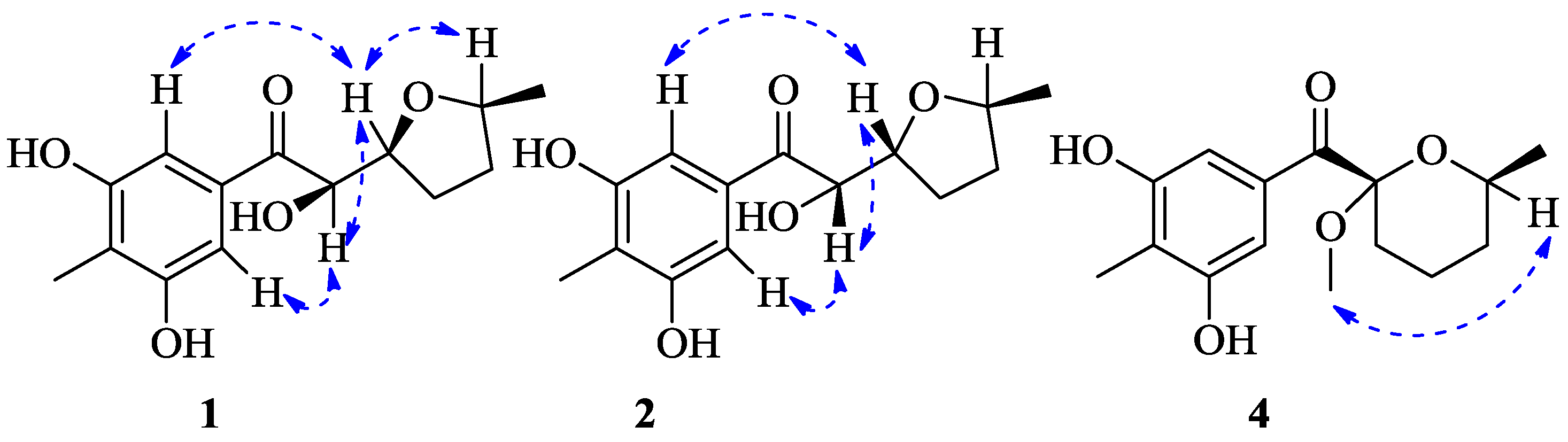
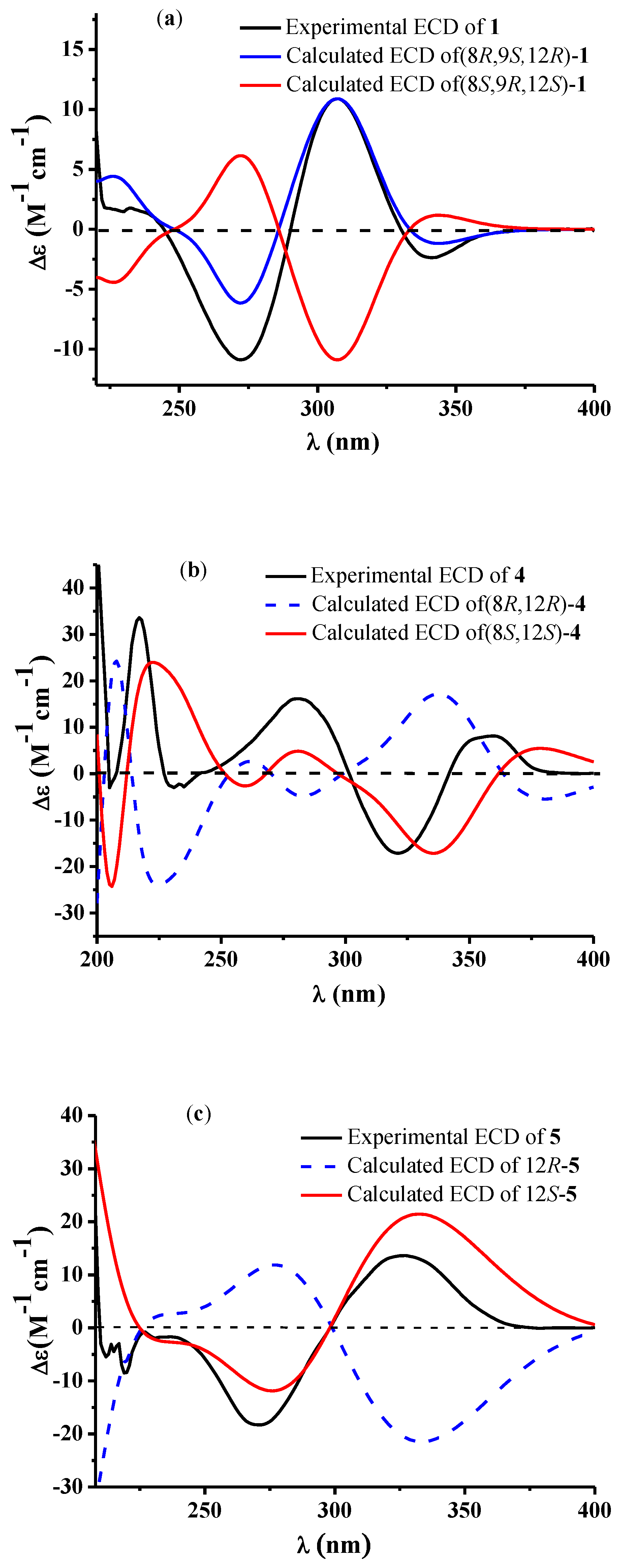
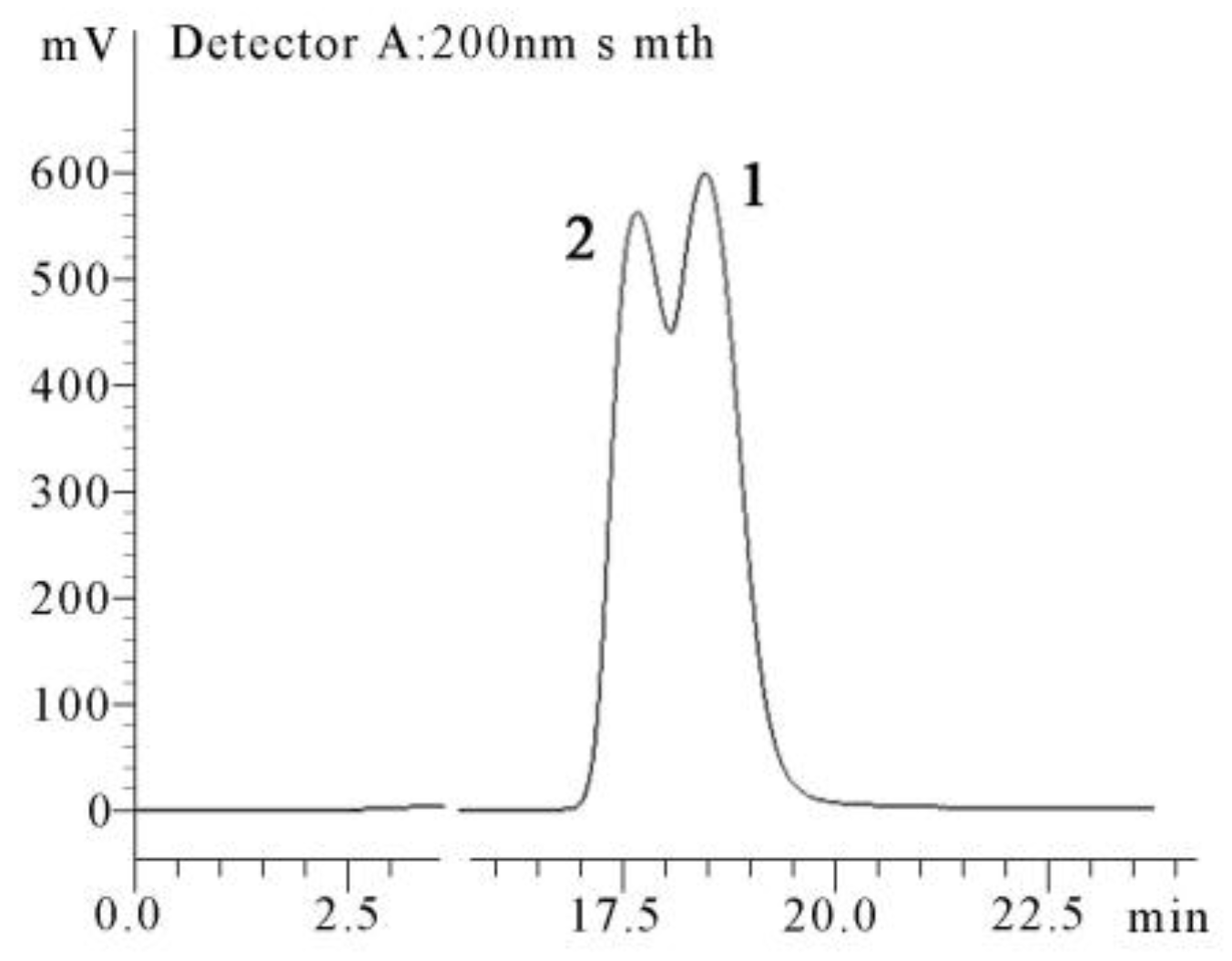
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Material
4.3. Fermentation, Extraction, and Isolation
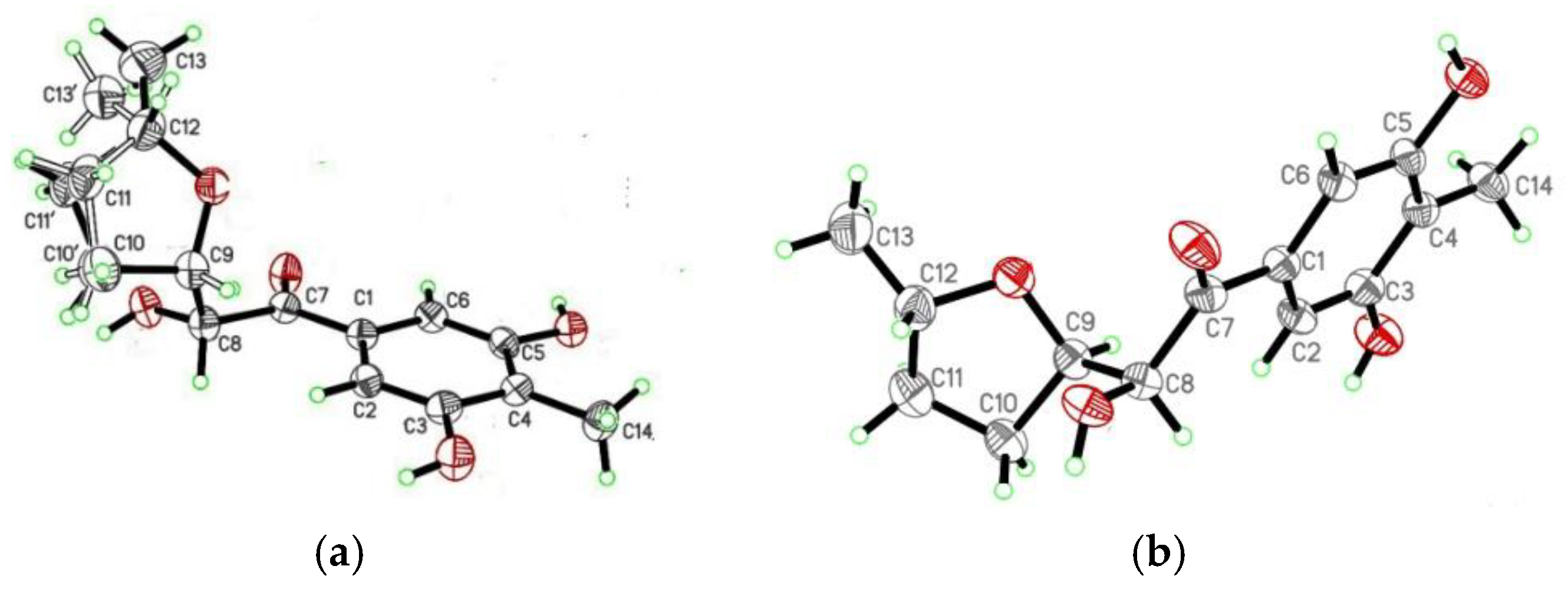
4.4. X-ray Crystallographic Analysis of the Diastereomeric Mixture of 1 and 2
4.5. Antioxidant Activity Assay
4.6. Cytotoxicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.S.; Yan, L.; Ma, L.Y.; Huang, Y.L.; Pan, X.H.; Liu, W.Z.; Lv, Z.H. Diphenyl derivatives from coastal saline soil fungus Aspergillus iizukae. Arch. Pharm. Res. 2015, 38, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.J.; Koch, M.; Abdel Aziz, M.H.; Galindo-Murillo, R.; Tianero, M.D.; Cheatham, T.E.; Barrows, L.R.; Reilly, C.A.; Schmidt, E.W. Oxazinin A, a pseudodimeric natural product of mixed biosynthetic origin from a filamentous fungus. Org. Lett. 2014, 16, 4774–4777. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Gunasekara, N.W.; Ratnaweera, P.B.; Zheng, Z.; Ellis, S.; Dada, S.; Patrick, B.O.; Wijesundera, R.L.C.; Nanayakkara, C.M.; Jefferies, W.A.; et al. Serpulanines A to C, N-oxidized tyrosine derivatives isolated from the Sri Lankan fungus Serpula sp.: Structure elucidation, synthesis, and histone deacetylase unhibition. J. Nat. Prod. 2018, 81, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.W.; Niu, S.B.; Li, L.; Geng, Z.F.; Liu, X.Z.; Che, Y.S. Identification of oxaphenalenone ketals from the Ascomycete fungus Neonectria sp. J. Nat. Prod. 2015, 78, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.C.; Grijseels, S.; Prigent, S.; Ji, B.; Dainat, J.; Nielsen, K.F.; Frisvad, J.C.; Workman, M.; Nielsen, J. Global analysis of biosynthetic gene clusters reveals vast potential of secondary metabolite production in Penicillium species. Nat. Microbiol. 2017, 2, 17044. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Tsai, K.J.S.; Harvey, C.J.B.; Li, J.J.; Ary, B.E.; Berlew, E.E.; Boehman, B.L.; Findley, D.M.; Friant, A.G.; Gardner, C.A.; et al. Comprehensive curation and analysis of fungal biosynthetic gene clusters of published natural products. Fungal Genet. Biol. 2016, 89, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, T.; Wang, M.; Li, L.; Si, J.; Song, B.; Zhou, C.; Yu, M.; Wang, X.; Zhang, Y.; Ding, G.; et al. Overexpression of the global regulator Laea in Chaetomium globosum leads to the biosynthesis of chaetoglobosin Z. J. Nat. Prod. 2016, 79, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Enghiad, B.; Zhao, H. New tools for reconstruction and heterologous expression of natural product biosynthetic gene clusters. Nat. Prod. Rep. 2016, 33, 174–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reen, F.J.; Romano, S.; Dobson, A.D.; O’Gara, F. The sound of silence: Activating silent biosynthetic gene clusters in marine microorganisms. Mar. Drugs 2015, 13, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Liu, Y.; Li, X.M.; Xu, G.M.; Ji, N.Y.; Wang, B.G. Citrifelins A and B, citrinin adducts with a tetracyclic framework from cocultures of marine-derived isolates of Penicillium citrinum and Beauveria felina. J. Nat. Prod. 2015, 78, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- He, X.Q.; Zhang, Z.Z.; Che, Q.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Varilactones and wortmannilactones produced by Penicillium variabile cultured with histone deacetylase inhibitor. Arch. Pharm. Res. 2018, 41, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Doull, J.L.; Singh, A.K.; Hoare, M.; Ayer, S.W. Conditions for the production of jadomycin B by Streptomyces venezuelae ISP5230: Effects of heat shock, ethanol treatment and phage infection. J. Ind. Microbiol. 1994, 13, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.H.; Wu, H.; Bai, L.Q.; Deng, Z.X.; Zhong, J.J. Temperature shift-induced reactive oxygen species enhanced validamycin A production in fermentation of Streptomyces hygroscopicus 5008. Bioprocess Biosyst. Eng. 2012, 35, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.Y.; Liu, D.S.; Li, D.G.; Huang, Y.L.; Kang, H.H.; Wang, C.H.; Liu, W.Z. Pyran rings containing polyketides from Penicillium raistrickii. Mar. Drugs 2017, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Ma, L.Y.; Liu, D.S.; Huang, Y.L.; Wang, C.H.; Shi, S.S.; Pan, X.H.; Song, X.D.; Zhu, R.X. Peniciketals A–C, new spiroketals from saline soil derived Penicillium raistrichii. Org. Lett. 2014, 16, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Liu, W.Z.; Shen, L.; Huang, Y.L.; Rong, X.G.; Xu, Y.Y.; Gao, X.D. Spiroketals, isocoumarin and indoleformic acid derivatives from saline soil derived fungus Penicillium raistrickii. Tetrahedron 2012, 68, 2276–2282. [Google Scholar] [CrossRef]
- Belofsky, G.N.; Gloer, K.B.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. New p-terphenyl and polyketide metabolites from the sclerotia of Penicillium raistrickii. J. Nat. Prod. 1998, 61, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.H.; Liu, D.S.; Wang, J.; Zhang, X.L.; Yan, M.M.; Zhang, D.H.; Zhang, J.J.; Liu, W.Z. Peneciraistin C induces caspase-independent autophagic cell death through mitochondrial-derived reactive oxygen species production in lung cancer cells. Cancer Sci. 2013, 104, 1476–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. Chembiochem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Karplus, M. Vicinal proton coupling in nuclear magnetic resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]
- Afifi, A.H.; Kagiyama, I.; El-Desoky, A.H.; Kato, H.; Mangindaan, R.E.P.; de Voogd, N.J.; Ammar, N.M.; Hifnawy, M.S.; Tsukamoto, S. Sulawesins A–C, furanosesterterpene tetronic acids that inhibit USP7, from a Psammocinia sp. marine sponge. J. Nat. Prod. 2017, 80, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Grkovic, T.; Pearce, A.N.; Munro, M.H.; Blunt, J.W.; Davies-Coleman, M.T.; Copp, B.R. Isolation and characterization of diastereomers of discorhabdins H and K and assignment of absolute configuration to discorhabdins D, N, Q, S, T, and U. J. Nat. Prod. 2010, 73, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; de Luna, R.D.; Cruz, W.C., Jr.; Rideout, J.A. Monoterpene lactones from the seeds of Nephelium lappaceum. J. Nat. Prod. 2005, 68, 1394–1396. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Andolfi, A.; Fiore, M.; Spanu, E.; Maddau, L.; Franceschini, A.; Marras, F.; Motta, A. Diplobifuranylones A and B, 5′-monosubstituted tetrahydro-2H-bifuranyl-5-ones produced by Diplodia corticola, a fungus pathogen of cork oak. J. Nat. Prod. 2006, 69, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.B.; Wang, S. Stereochemistry of the roridins. diastereomers of roridin E. J. Nat. Prod. 1999, 62, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Presley, C.C.; Valenciano, A.L.; Fernández-Murga, M.L.; Du, Y.; Shanaiah, N.; Cassera, M.B.; Goetz, M.; Clement, J.A.; Kingston, D.G.I. Antiplasmodial chromanes and chromenes from the monotypic plant species Koeberlinia spinosa. J. Nat. Prod. 2018, 81, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Thoison, O.; Fahy, J.; Dumontet, V.; Chiaroni, A.; Riche, C.; Tri, M.V.; Sévenet, T. Cytotoxic prenylxanthones from Garcinia bracteata. J. Nat. Prod. 2000, 63, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Boonnak, N.; Chantrapromma, S.; Fun, H.K.; Yuenyongsawad, S.; Patrick, B.O.; Maneerat, W.; Williams, D.E.; Andersen, R.J. Three types of cytotoxic natural caged-scaffolds: Pure enantiomers or partial racemates. J. Nat. Prod. 2014, 77, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Liu, W.Z.; Huang, Y.L.; Rong, X.G. Two acid sorbicillin analogues from saline lands-derived fungus Trichoderma sp. J. Antibiot. 2011, 64, 645–647. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 133.0, C | 132.9, C | 133.5, C | |||
| 2, 6 | 106.2, CH | 6.90, s | 106.2, CH | 6.89, s | 106.2, CH | 6.94, s |
| 3, 5 | 156.2, C | 156.2, C | 156.3, C | |||
| 4 | 116.6, C | 116.6, C | 116.7, C | |||
| 7 | 199.0, C | 199.2, C | 199.4, C | |||
| 8 | 74.9, CH | 4.74, dd (7.3, 3.5) | 75.4, CH | 4.69, dd (7.3, 3.3) | 74.8, CH | 4.73, t (5.8) |
| 9 | 80.1, CH | 4.13, m | 79.5, CH | 4.27, td (7.0, 3.3) | 79.3, CH | 4.20, q (5.8) |
| 10 | 27.3, CH2 | 1.84, a m | 27.8, CH2 | 1.93, a m | 26.5, CH2 | 1.84, m; 1.77, m |
| 11 | 32.5, CH2 | 1.84, a m; 1.34, m | 33.4, CH2 | 1.93, a m; 1.31, m | 33.1, CH2 | 1.99, m; 1.34, m |
| 12 | 75.2, CH | 3.77, m | 75.4, CH | 3.99, m | 75.0, CH | 4.04, m |
| 13 | 20.6, CH3 | 1.06, d (6.0) | 21.0, CH3 | 1.02, d (6.1) | 21.0, CH3 | 1.04, d (6.0) |
| 14 | 8.9, CH3 | 1.99, s | 8.9, CH3 | 1.99, s | 8.9, CH3 | 1.99, s |
| 15 | ||||||
| OH-3, 5 | 9.51, s | 9.52, s | 9.49, s | |||
| OH-8 | 4.84, d (7.3) | 4.96, d (7.3) | 5.31, d (5.8) | |||
| Position | 4 a | 5 a | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 133.9, C | 136.3, C | ||
| 2, 6 | 109.2, CH | 7.36, s | 108.7, CH | 6.94, s |
| 3, 5 | 156.8, C | 156.8, C | ||
| 4 | 117.6, C | 116.8, C | ||
| 7 | 196.5, C | 190.1, C | ||
| 8 | 102.6, C | 152.7, C | ||
| 9 | 32.3, CH2 | 1.80, m; 1.65, b m | 112.2, CH | 5.71, t (3.8) |
| 10 | 19.7, CH2 | 1.91, m; 1.65, b m | 21.6, CH2 | 2.30, m; 2.20, m |
| 11 | 32.7, CH2 | 1.65, b m; 1.40, m | 29.3, CH2 | 1.94, m; 1.55, m |
| 12 | 68.0, CH | 3.88, m | 73.0, CH | 4.04, m |
| 13 | 22.0, CH3 | 1.22, d (6.3) | 21.2, CH3 | 1.32, d (6.2) |
| 14 | 9.0, CH3 | 2.13, s | 9.0, CH3 | 2.15, s |
| 15 | 50.5, CH3 | 3.17, s | ||
| OH-3, 5 | 8.43, s | 8.45, s | ||
| OH-8 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.-S.; Rong, X.-G.; Kang, H.-H.; Ma, L.-Y.; Hamann, M.T.; Liu, W.-Z. Raistrickiones A−E from a Highly Productive Strain of Penicillium raistrickii Generated through Thermo Change. Mar. Drugs 2018, 16, 213. https://doi.org/10.3390/md16060213
Liu D-S, Rong X-G, Kang H-H, Ma L-Y, Hamann MT, Liu W-Z. Raistrickiones A−E from a Highly Productive Strain of Penicillium raistrickii Generated through Thermo Change. Marine Drugs. 2018; 16(6):213. https://doi.org/10.3390/md16060213
Chicago/Turabian StyleLiu, De-Sheng, Xian-Guo Rong, Hui-Hui Kang, Li-Ying Ma, Mark T. Hamann, and Wei-Zhong Liu. 2018. "Raistrickiones A−E from a Highly Productive Strain of Penicillium raistrickii Generated through Thermo Change" Marine Drugs 16, no. 6: 213. https://doi.org/10.3390/md16060213




