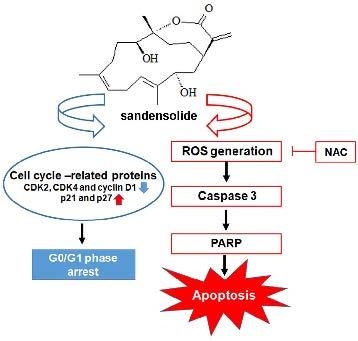
Sandensolide Induces Oxidative Stress-Mediated Apoptosis in Oral Cancer Cells and in Zebrafish Xenograft Model
Abstract
1. Introduction
2. Results
2.1. Effect of Sandensolide on the Cell Viability of Oral Cancer Cells
2.2. Effect of Sandensolide on Cell Cycle Arrest of Oral Cancers
2.3. Effect of Sandensolide on Apoptosis of Oral Cancers
2.4. Effect of Sandensolide on ROS generation of Oral Cancers
2.5. Effect of Sandensolide on ROS-Mediated Caspases-Based Apoptosis of Oral Cancers
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Lines and Culture
4.3. Cell Viability
4.4. Colony Formation Assay
4.5. Cell Cycle Analysis
4.6. Intracellular ROS Determination
4.7. Western Blot Analysis
4.8. Zebrafish Xenograft Assay
4.9. Zebrafish Xenograft Assay
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- De Silva, R.K.; Siriwardena, B.; Samaranayaka, A.; Abeyasinghe, W.; Tilakaratne, W.M. A model to predict nodal metastasis in patients with oral squamous cell carcinoma. PLoS ONE 2018, 13, e0201755. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Johnson, N.W.; Kumar, N. Global epidemiology of head and neck cancers: A continuing challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.F.; Lin, C.S.; Chen, Y.H.; Sung, P.J.; Lin, S.R.; Tong, Y.W.; Weng, C.F. Inhibitory growth of oral squamous cell carcinoma cancer via bacterial prodigiosin. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Katakwar, P.; Metgud, R.; Naik, S.; Mittal, R. Oxidative stress marker in oral cancer: A review. J. Cancer. Res. Ther. 2016, 12, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.B.; de Araujo, T.B.S.; de Freitas, R.D.; Rodrigues, A.; Sousa, L.P.; Sales, C.B.S.; Valverde, L.F.; Soares, M.B.P.; Dos Reis, M.G.; Coletta, R.D.; et al. Beta-Lapachone and its iodine derivatives cause cell cycle arrest at G2/M phase and reactive oxygen species-mediated apoptosis in human oral squamous cell carcinoma cells. Free Radic. Biol. Med. 2018, 126, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Wu, C.Y.; Tang, J.Y.; Huang, C.Y.; Liaw, C.C.; Wu, S.H.; Sheu, J.H.; Chang, H.W. Sinularin induces oxidative stress-mediated G2/M arrest and apoptosis in oral cancer cells. Environ. Toxicol. 2017, 32, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.S.; Tang, J.Y.; Yen, C.Y.; Huang, H.W.; Wu, C.Y.; Chung, Y.A.; Wang, H.R.; Chen, I.S.; Huang, M.Y.; Chang, H.W. Antiproliferation of Cryptocarya concinna-derived cryptocaryone against oral cancer cells involving apoptosis, oxidative stress, and DNA damage. BMC Complemt. Altern. Med. 2016, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.T.; Kuo, C.L.; Chueh, F.S.; Liu, K.C.; Bau, D.T.; Chung, J.G. Curcuminoids induce reactive oxygen species and autophagy to enhance apoptosis in human oral cancer cells. Am. J. Chin. Med. 2018, 46, 1145–1168. [Google Scholar] [CrossRef] [PubMed]
- Sithranga, B.N.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kang, K.A.; Piao, M.J.; Kumara, M.H.; Jeong, Y.J.; Ko, M.H.; Hyun, J.W. Generation of reactive oxygen species and endoplasmic reticulum stress by dictyopteris undulata extract leads to apoptosis in human melanoma cells. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Huang, C.Y.; Tang, J.Y.; Liaw, C.C.; Li, R.N.; Liu, J.R.; Sheu, J.H.; Chang, H.W. Reactive oxygen species mediate soft corals-derived sinuleptolide-induced antiproliferation and DNA damage in oral cancer cells. Onco. Targets Ther. 2017, 10, 3289–3297. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Koganemaru, R.; Iwakawa, Y.; Higuchi, R.; Miyamoto, T. Inhibitory effect of norditerpenes on LPS-induced TNF-alpha production from the Okinawan soft coral, Sinularia sp. Biol. Pharm. Bull. 2003, 26, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H.; Wang, G.H.; Chou, T.H.; Wang, S.H.; Lin, R.J.; Chan, L.P.; So, E.C.; Sheu, J.H. 5-epi-Sinuleptolide induces cell cycle arrest and apoptosis through tumor necrosis factor/mitochondria-mediated caspase signaling pathway in human skin cancer cells. Biochim. Biophys. Acta 2012, 1820, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.C.; Huang, Y.T.; Chou, S.K.; Shih, M.C.; Chiang, C.Y.; Su, J.H. Cytotoxic oxygenated steroids from the soft coral nephthea erecta. Chem. Pharm. Bull (Tokyo) 2016, 64, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Chiu, C.F.; Hu, J.L.; Feng, C.H.; Huang, C.Y.; Bai, L.Y.; Sheu, J.H. A sterol from soft coral induces apoptosis and autophagy in MCF-7 breast cancer cells. Mar. Drugs 2018, 16, 238. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Kao, C.L.; Li, H.T.; Chen, C.Y. Chemical constituents of cultured soft coral Sinularia flexibilis. Chem. Nat. Compd. 2018, 54, 168–169. [Google Scholar] [CrossRef]
- Cuzick, J. Preventive therapy for cancer. Lancet. Oncol. 2017, 18, e472–e482. [Google Scholar] [CrossRef]
- Goyal, S.; Gupta, N.; Chatterjee, S.; Nimesh, S. Natural plant extracts as potential therapeutic agents for the treatment of cancer. Curr. Top. Med. Chem. 2017, 17, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Yan, Y.; Li, S.; Zhao, L.; Zhang, C.; Liu, L.; Wang, C. The in vitro anti-tumor activity of phycocyanin against non-small cell lung cancer cells. Mar. Drugs 2018, 16, 178. [Google Scholar] [CrossRef] [PubMed]
- Castro-Carvalho, B.; Ramos, A.A.; Prata-Sena, M.; Malhao, F.; Moreira, M.; Gargiulo, D.; Dethoup, T.; Buttachon, S.; Kijjoa, A.; Rocha, E. Marine-derived fungi extracts enhance the cytotoxic activity of doxorubicin in nonsmall cell lung cancer cells A459. Pharmacogn. Res. 2017, 9, S92–S98. [Google Scholar]
- Sawadogo, W.R.; Boly, R.; Cerella, C.; Teiten, M.H.; Dicato, M.; Diederich, M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2012. Molecules 2015, 20, 7097–7142. [Google Scholar] [CrossRef] [PubMed]
- Aspeslagh, S.; Stein, M.; Bahleda, R.; Hollebecque, A.; Salles, G.; Gyan, E.; Fudio, S.; Extremera, S.; Alfaro, V.; Soto-Matos, A.; et al. Phase I dose-escalation study of plitidepsin in combination with sorafenib or gemcitabine in patients with refractory solid tumors or lymphomas. Anticancer Drugs 2017, 28, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Shirley, H.J.; Jamieson, M.L.; Brimble, M.A.; Bray, C.D. A new family of sesterterpenoids isolated around the Pacific Rim. Nat. Prod. Rep. 2018, 35, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chao, C.H.; Huang, T.Z.; Huang, C.Y.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Cembranoid-related metabolites and biological activities from the soft coral sinularia flexibilis. Mar. Drugs 2018, 16, 278. [Google Scholar] [CrossRef] [PubMed]
- Piccinetti, C.C.; Ricci, R.; Pennesi, C.; Radaelli, G.; Totti, C.; Norici, A.; Giordano, M.; Olivotto, I. Herbivory in the soft coral Sinularia flexibilis (Alcyoniidae). Sci. Rep. 2016, 6, 22679. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Liu, H.L.; Yao, L.G.; Guo, Y.W. 9,11-Secosteroids and polyhydroxylated steroids from two South China Sea soft corals Sarcophyton trocheliophorum and Sinularia flexibilis. Steroids 2014, 92, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.C.; Yen, W.H.; Su, J.H.; Chiang, M.Y.; Wen, Z.H.; Chen, W.F.; Lu, T.J.; Chang, Y.W.; Chen, Y.H.; Wang, W.H.; et al. Cembrane derivatives from the soft corals, Sinularia gaweli and Sinularia flexibilis. Mar. Drugs 2013, 11, 2154–2167. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.C.; Su, J.H.; Chiang, M.Y.; Lu, M.C.; Hwang, T.L.; Chen, Y.H.; Hu, W.P.; Lin, N.C.; Wang, W.H.; Fang, L.S.; et al. Flexibilins A-C, new cembrane-type diterpenoids from the Formosan soft coral, Sinularia flexibilis. Mar. Drugs 2013, 11, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Kuo, C.Y.; Wen, Z.H.; Lin, Y.Y.; Wang, W.H.; Su, J.H.; Sheu, J.H.; Sung, P.J. Flexibilisquinone, a new anti-inflammatory quinone from the cultured soft coral Sinularia flexibilis. Molecules 2013, 18, 8160–8167. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.W.; Hung, H.C.; Huang, S.Y.; Chen, C.H.; Chen, Y.R.; Chen, C.Y.; Yang, S.N.; Wang, H.D.; Sung, P.J.; Sheu, J.H.; et al. Neuroprotective effect of the marine-derived compound 11-dehydrosinulariolide through DJ-1-related pathway in vitro and in vivo models of parkinson’s disease. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Su, J.H.; Lin, Y.F.; Lu, Y.; Yeh, H.C.; Wang, W.H.; Fan, T.Y.; Sheu, J.H. Oxygenated cembranoids from the cultured and wild-type soft corals Sinularia flexibilis. Chem. Pharm. Bull (Tokyo) 2009, 57, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Wong, B.S.; Yea, S.H.; Lu, C.I.; Weng, S.H. Sinularin induces apoptosis through mitochondria dysfunction and inactivation of the pI3K/Akt/mTOR pathway in gastric carcinoma cells. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C.; Din, Z.H.; Su, J.H.; Wu, Y.J.; Liu, C.I. Sinulariolide suppresses cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 and Urokinase through the PI3K/AKT/mTOR signaling pathway in human bladder cancer cells. Mar. Drugs 2017, 15, 238. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Neoh, C.A.; Tsao, C.Y.; Su, J.H.; Li, H.H. Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through MAPKs and PI3K/Akt signaling pathways. Int. J. Mol. Sci. 2015, 16, 16469–16482. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Su, J.H.; Chiu, C.C.; Lin, J.J.; Yang, Z.Y.; Hwang, W.I.; Chen, Y.K.; Lo, Y.H.; Wu, Y.J. Proteomic investigation of the sinulariolide-treated melanoma cells A375: Effects on the cell apoptosis through mitochondrial-related pathway and activation of caspase cascade. Mar. Drugs 2013, 11, 2625–2642. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Lin, S.C.; Su, J.H.; Chen, Y.K.; Lin, C.C.; Chan, H.L. Sinularin induces DNA damage, G2/M phase arrest, and apoptosis in human hepatocellular carcinoma cells. BMC Complemt. Altern. Med. 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Teoh, S.L.; Das, S.; Mahakknaukrauh, P. Oxidative stress in oral diseases: Understanding its relation with other systemic diseases. Front. Physiol. 2017, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.S.; Sharma, A.K.; Soni, H.; Ali, D.M.; Tews, B.; Konig, R.; Eibl, H.; Berger, M.R. Induction of ER and mitochondrial stress by the alkylphosphocholine erufosine in oral squamous cell carcinoma cells. Cell Death Dis. 2018, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015, 4, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Luna-Dulcey, L.; Tomasin, R.; Naves, M.A.; da Silva, J.A.; Cominetti, M.R. Autophagy-dependent apoptosis is triggered by a semi-synthetic [6]-gingerol analogue in triple negative breast cancer cells. Oncotarget 2018, 9, 30787–30804. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chu, C.S.; Tsai, H.J.; Ke, L.Y.; Lee, H.C.; Yeh, J.L.; Chen, C.H.; Wu, B.N. Xanthine-derived KMUP-1 reverses glucotoxicity-activated Kv channels through the cAMP/PKA signaling pathway in rat pancreatic beta cells. Chem. Biol. Interact. 2018, 279, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.C.; Lo, S.; Hou, M.F.; Lee, Y.C.; Tsai, C.H.; Chen, Y.Y.; Liu, W.; Su, Y.H.; Lo, Y.H.; Wang, C.H.; et al. Extracellular visfatin-promoted malignant behavior in breast cancer is mediated through c-Abl and STAT3 activation. Clin. Cancer Res. 2016, 22, 4478–4490. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Rouhi, P.; Dahl Jensen, L.; Zhang, D.; Ji, H.; Hauptmann, G.; Ingham, P.; Cao, Y. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc. Natl. Acad Sci. USA 2009, 106, 19485–19490. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.-I.; Chen, C.-Y.; Liu, W.; Chang, P.-C.; Huang, C.-W.; Han, K.-F.; Lin, I.-P.; Lin, M.-Y.; Lee, C.-H. Sandensolide Induces Oxidative Stress-Mediated Apoptosis in Oral Cancer Cells and in Zebrafish Xenograft Model. Mar. Drugs 2018, 16, 387. https://doi.org/10.3390/md16100387
Yu C-I, Chen C-Y, Liu W, Chang P-C, Huang C-W, Han K-F, Lin I-P, Lin M-Y, Lee C-H. Sandensolide Induces Oxidative Stress-Mediated Apoptosis in Oral Cancer Cells and in Zebrafish Xenograft Model. Marine Drugs. 2018; 16(10):387. https://doi.org/10.3390/md16100387
Chicago/Turabian StyleYu, Chung-I, Chung-Yi Chen, Wangta Liu, Po-Chih Chang, Chiung-Wei Huang, Kuang-Fen Han, In-Pin Lin, Mei-Ying Lin, and Chien-Hsing Lee. 2018. "Sandensolide Induces Oxidative Stress-Mediated Apoptosis in Oral Cancer Cells and in Zebrafish Xenograft Model" Marine Drugs 16, no. 10: 387. https://doi.org/10.3390/md16100387
APA StyleYu, C.-I., Chen, C.-Y., Liu, W., Chang, P.-C., Huang, C.-W., Han, K.-F., Lin, I.-P., Lin, M.-Y., & Lee, C.-H. (2018). Sandensolide Induces Oxidative Stress-Mediated Apoptosis in Oral Cancer Cells and in Zebrafish Xenograft Model. Marine Drugs, 16(10), 387. https://doi.org/10.3390/md16100387