Anticancer Activity of Fascaplysin against Lung Cancer Cell and Small Cell Lung Cancer Circulating Tumor Cell Lines
Abstract
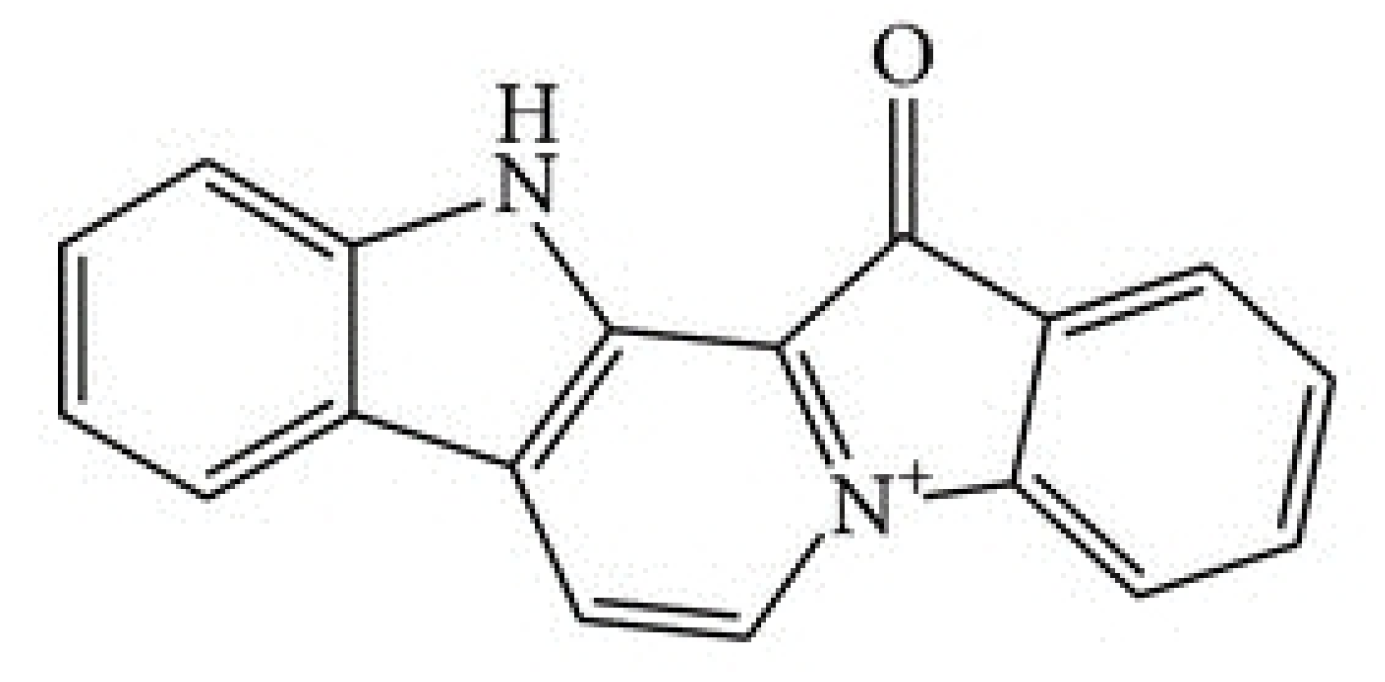
:1. Introduction
2. Results
2.1. Fascaplysin Cytotoxicity against SCLC, NSCLC and Non-lung Cancer Cell Lines
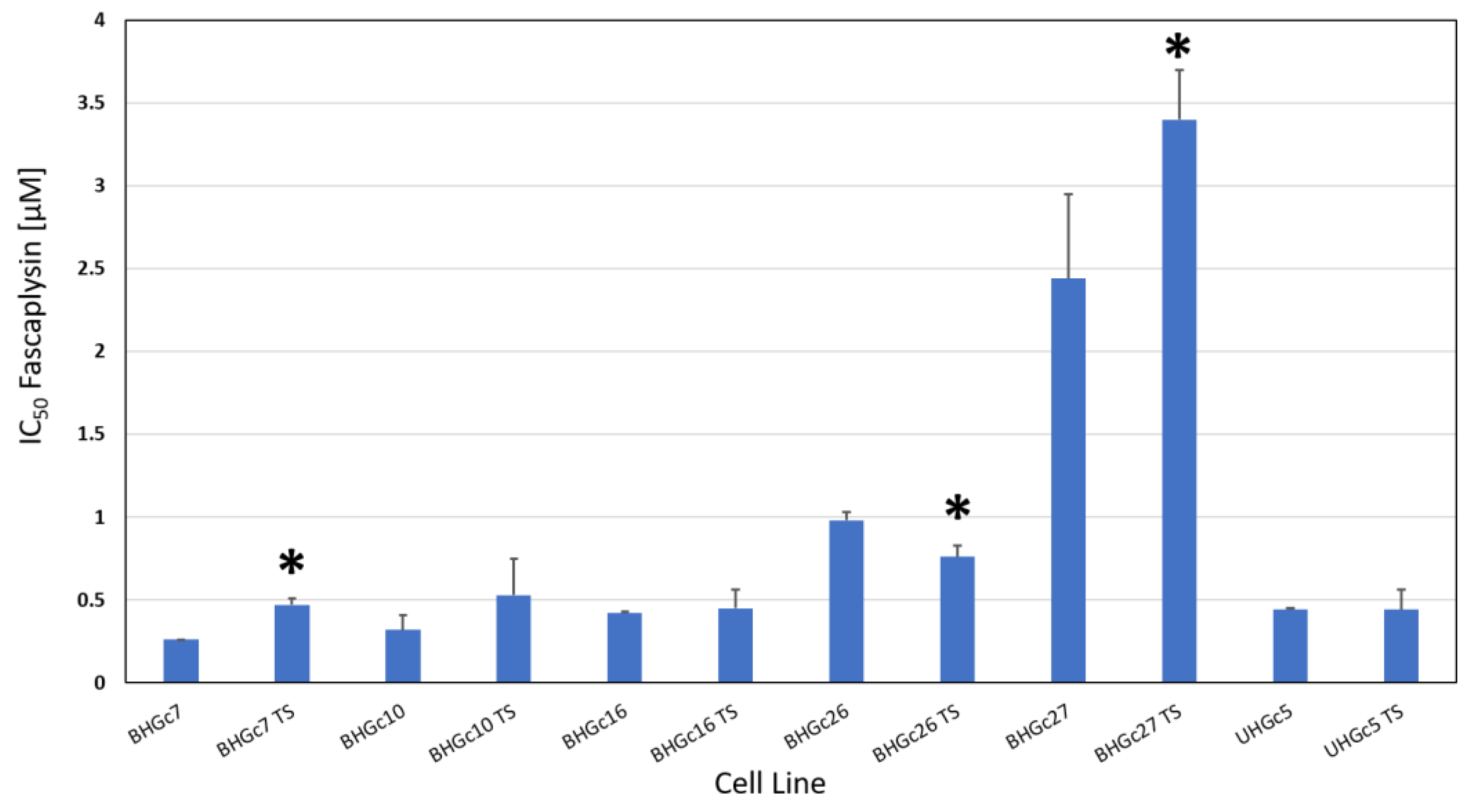
2.2. Fascaplysin Cytotoxicity against SCLC CTC Single Cells and Tumorospheres
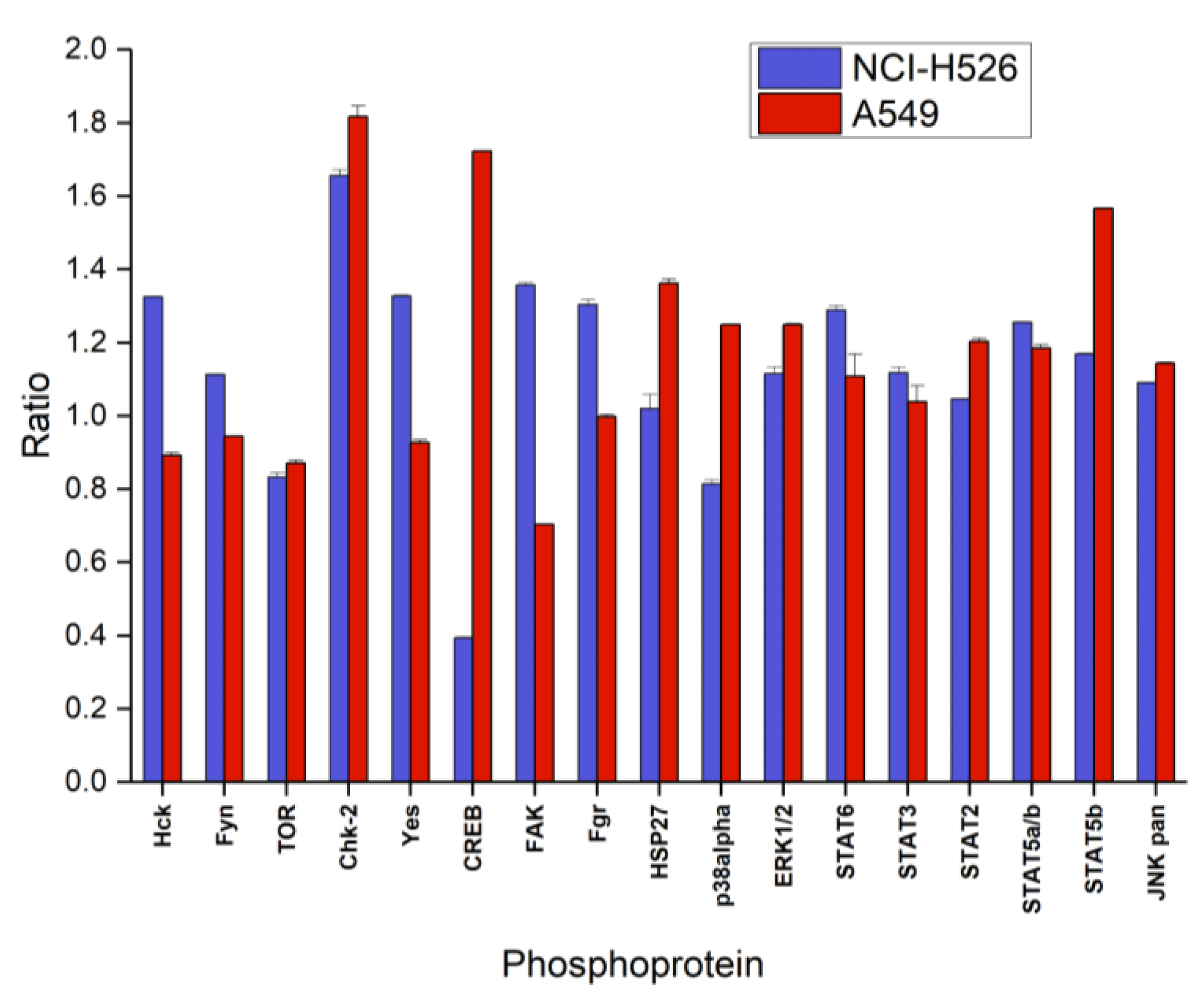
2.3. Alterations of Selected Phosphoproteins of NCI-H526 and A549 in Response to Fascaplysin
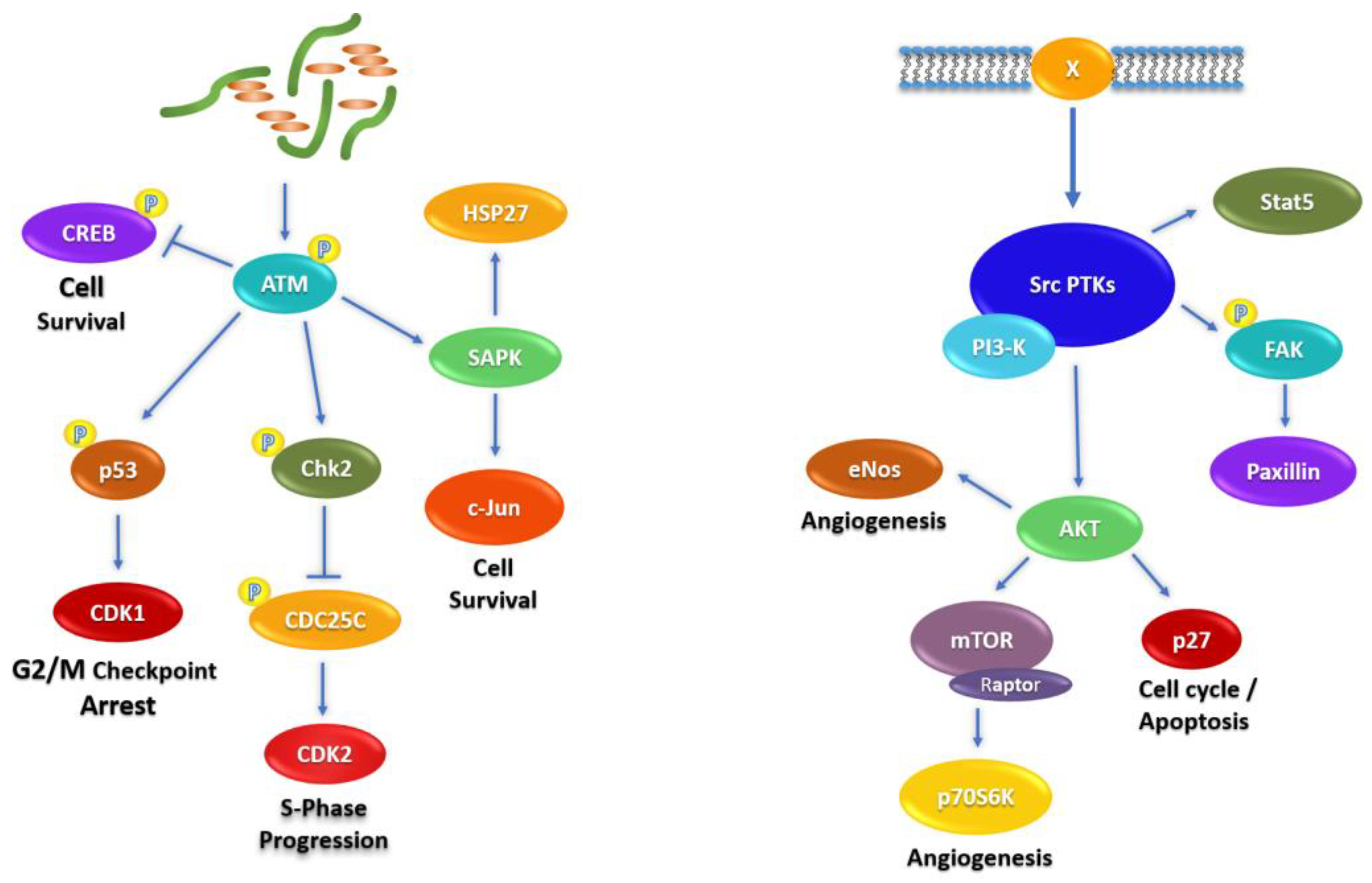
2.4. Signaling Pathways Affected by Fascaplysin in NCI-H526 and A549 Lung Cancer Cells
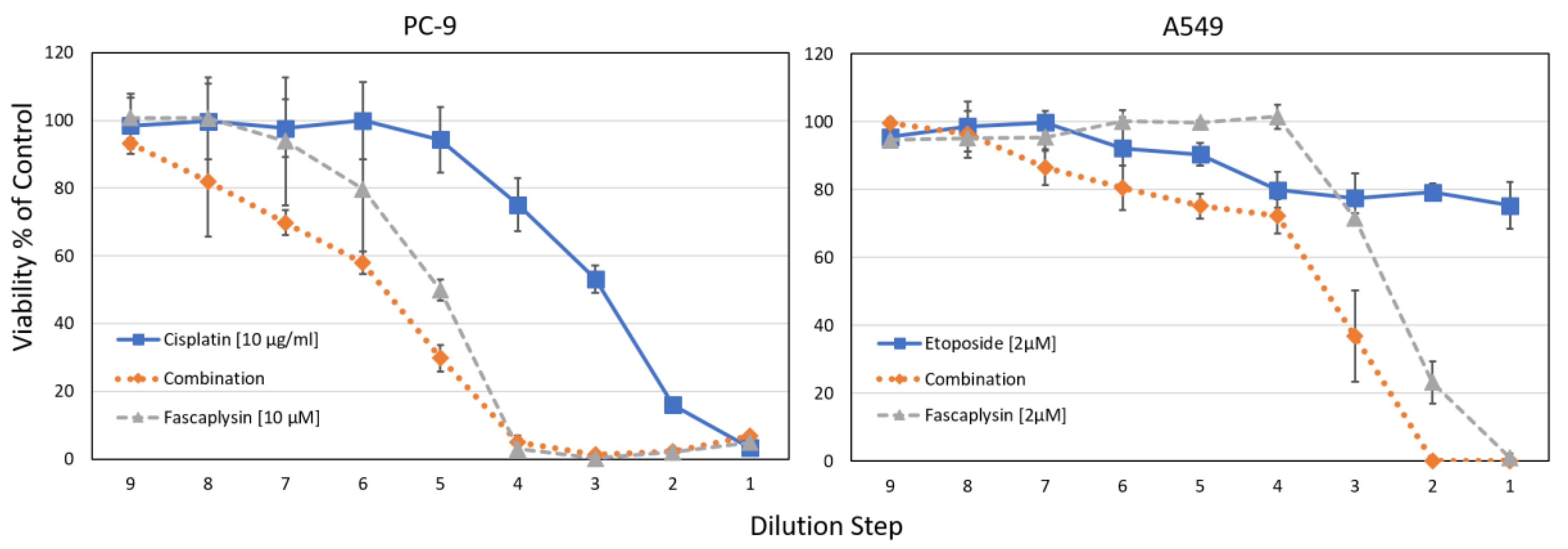
2.5. Combination of Cisplatin/Etoposide with Fascaplysin in Cytotoxicity Assays for NCI-H526 and A549 Cell Lines
3. Discussion
4. Cell Culture and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Phosphokinase Array
4.4. Cytotoxicity Assay
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [PubMed]
- Kalemkerian, G.P. Small Cell Lung Cancer. Semin. Respir. Crit. Care Med. 2016, 37, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Current status of targeted therapy in non-small cell lung cancer. Drugs Today (Barc) 2014, 50, 503–525. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.S.; Neal, J.W.; Wakelee, H. Review of the current targeted therapies for non-small-cell lung cancer. World J. Clin. Oncol. 2014, 5, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; Summers, Y.; Cadranel, J.; Benepal, T.; Christoph, D.C.; Lal, R.; Das, M.; Maxwell, F.; Visseren-Grul, C.; Ferry, D. Cisplatin in the modern era: The backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat. Rev. 2016, 44, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Klameth, L.; Rath, B.; Hochmaier, M.; Moser, D.; Redl, M.; Mungenast, F.; Gelles, K.; Ulsperger, E.; Zeillinger, R.; Hamilton, G. Small cell lung cancer: model of circulating tumor cell tumorospheres in chemoresistance. Sci. Rep. 2017, 7, 5337. [Google Scholar] [CrossRef] [PubMed]
- Roll, D.M.; Ireland, C.M.; Lu, H.S.M.; Clardy, J. Fascaplysin, an unusual antimicrobial pigment from the marine sponge Fascaplysinopsis sp. J. Org. Chem. 1988, 53, 3276–3278. [Google Scholar] [CrossRef]
- Segraves, N.L.; Robinson, S.J.; Garcia, D.; Said, S.A.; Fu, X.; Schmitz, F.J.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Comparison of fascaplysin and related alkaloids: A study of structures, cytotoxicities, and sources. J. Nat. Prod. 2004, 67, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Segraves, N.L.; Lopez, S.; Johnson, T.A.; Said, S.A.; Fu, X.; Schmitz, F.J.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Structures and cytotoxicities of fascaplysin and related alkaloids from two marine phyla—Fascaplysinopsis sponges and Didemnum tunicates. Tetrahedron Lett. 2003, 44, 3471–3475. [Google Scholar] [CrossRef]
- Bharate, S.B.; Manda, S.; Mupparapu, N.; Battini, N.; Vishwakarma, R.A. Chemistry and biology of fascaplysin, a potent marine-derived CDK-4 inhibitor. Mini Rev. Med. Chem. 2012, 12, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Muller, L.; Furet, P.; Schoepfer, J.; Stephan, C.; Zumstein-Mecker, S.; Fretz, H.; Chaudhuri, B. Inhibition of cyclindependentkinase 4 (Cdk4) by fascaplysin, a marine natural product. Biochem. Biophys. Res. Commun. 2000, 275, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; O’Reilly, T.; Furet, P.; Muller, L.; Stephan, C.; Zumstein-Mecker, S.; Fretz, H.; Fabbro, D.; Chaudhuri, B. Selective in vivo and in vitro effects of a small molecule inhibitor of cyclindependent kinase-4. J. Natl. Cancer Inst. 2001, 93, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.I.; Steinbrecher, T.; Schmid, R. Fascaplysin as a specific inhibitor for CDK4: Insights from molecular modelling. PLoS ONE 2012, 7, e42612. [Google Scholar] [CrossRef] [PubMed]
- Hormann, A.; Chaudhari, B.; Fretz, H. DNA binding properties of marine sponge pigment fascaplysin. Bioorg. Med. Chem. 2001, 9, 917–921. [Google Scholar] [CrossRef]
- Mahale, S.; Bharate, S.B.; Manda, S.; Joshi, P.; Bharate, S.S.; Jenkins, P.R.; Vishwakarma, R.A.; Chaudhuri, B. Biphenyl-4-carboxylic Acid [2-(1H-Indol-3-yl)-ethyl]-methylamide (CA224), a nonplanar analogue of Fascaplysin, inhibits Cdk4 and tubulin polymerization: evaluation of in vitro and in vivo anticancer activity. J. Med. Chem. 2014, 57, 9658–9672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar Guru, S.; Pathania, A.S.; Manda, S.; Kumar, A.; Bharate, S.B.; Vishwakarma, R.A.; Malik, F.; Bhushan, S. Fascaplysin induces caspase mediated crosstalk between apoptosis and autophagy through the inhibition of PI3K/AKT/mTOR signaling cascade in human leukemia HL-60 cells. J. Cell Biochem. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chen, H.; Lu, X.; Wang, F.; Xu, W.; Jin, H.; Zhu, P. Fascaplysin exert anti-tumor effects through apoptotic and anti-angiogenesis pathways in sarcoma mice model. Eur. J. Pharm. Sci. 2011, 43, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.L.; Lu, X.L.; Lin, J.; Chen, H.M.; Yan, X.J.; Wang, F.; Xu, W.F. Direct effects of fascaplysin on human umbilical vein endothelial cells attributing the anti-angiogenesis activity. Biomed. Pharmacother. 2010, 64, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G. Cytotoxic effects of fascaplysin against small cell lung cancer cell lines. Mar Drugs 2014, 12, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.; Olszewski, U.; Klameth, L.; Ulsperger, E.; Geissler, K. Synergistic anticancer activity of topotecan—Cyclin-dependent kinase inhibitor combinations against drug-resistant small cell lung cancer (SCLC) cell lines. J. Cancer Ther. 2013, 4, 47–53. [Google Scholar] [CrossRef]
- Hamilton, G.; Klameth, L.; Rath, B.; Thalhammer, T. Synergism of cyclin-dependent kinase inhibitors with camptothecin derivatives in small cell lung cancer cell lines. Molecules 2014, 19, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Aarts, M.; Linardopoulos, S.; Turner, N.C. Tumor selective targeting of cell cycle kinases for cancer treatment. Curr. Opin. Pharmacol. 2013, 13, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.R.; Mallinger, A.; Workman, P.; Clarke, P.A. Inhibitors of cyclin-dependent kinases as cancer therapeutics. Pharmacol. Ther. 2017, 173, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Graf, F.; Mosch, B.; Koehler, L.; Bergmann, R.; Wuest, F.; Pietzsch, J. Cyclin-Dependent Kinase 4/6 (Cdk4/6) Inhibitors: Perspectives in Cancer Therapy and Imaging. Mini Rev. Med. Chem. 2015, 15. in press. [Google Scholar] [CrossRef]
- Jaganathan, H.; Overstreet, K.; Reed, E. Improving breast cancer therapy with CDK4/6 inhibitors. Clin. Breast Cancer 2014, 14, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Beasley, M.B.; Lantuejoul, S.; Abbondanzo, S.; Chu, W.S.; Hasleton, P.S.; Travis, W.D.; Brambilla, E. The P16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung. Hum. Pathol. 2003, 34, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I.; Edwards, C.D.; Kobzik, L.; Godleski, J.; Richards, W.; Sugarbaker, D.J.; Rollins, B.J. Reciprocal Rb inactivation and p16INK4 expression in primary lung cancers and cell lines. Cancer Res. 1995, 55, 505–509. [Google Scholar] [PubMed]
- Coe, BP.; Lockwood, W.W.; Girard, L.; Chari, R.; Macaulay, C.; Lam, S.; Gazdar, A.F.; Minna, J.D.; Lam, W.L. Differential disruption of cell cycle pathways in small cell and non-small cell lung cancer. Br. J. Cancer 2006, 94, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. The DNA damage response: making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, A.T.; Kim, S.H.; Chang, H.Y.; Mastrocola, A.S.; Tibbetts, R.S. Cyclin-dependent kinase 1-dependent phosphorylation of cAMP response element-binding protein decreases chromatin occupancy. J. Biol. Chem. 2013, 288, 23765–23775. [Google Scholar] [CrossRef] [PubMed]
- Rolli, M.; Kotlyarov, A.; Sakamoto, K.M.; Gaestel, M.; Neininger, A. Stress-induced stimulation of early growth response gene-1 by p38/stress-activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2-independent manner. J. Biol. Chem. 1999, 274, 19559–19564. [Google Scholar] [CrossRef] [PubMed]
- Villedieu, M.; Deslandes, E.; Duval, M.; Héron, J.F.; Gauduchon, P.; Poulain, L. Acquisition of chemoresistance following discontinuous exposures to cisplatin is associated in ovarian carcinoma cells with progressive alteration of FAK, ERK and p38 activation in response to treatment. Gynecol. Oncol. 2006, 101, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Warmuth, M.; Damoiseaux, R.; Liu, Y.; Fabbro, D.; Gray, N. SRC family kinases: potential targets for the treatment of human cancer and leukemia. Curr. Pharm. Des. 2003, 9, 2043–2059. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.; Kopetz, S. The SRC family of protein tyrosine kinases: a new and promising target for colorectal cancer therapy. Clin. Colorectal Cancer 2010, 9, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Silvers, A.L.; Bachelor, M.A.; Bowden, G.T. The role of JNK and p38 MAPK activities in UVA-induced signaling pathways leading to AP-1 activation and c-Fos expression. Neoplasia 2003, 5, 319–329. [Google Scholar] [CrossRef]
- Gao, J.; Wagnon, J.L.; Protacio, R.M.; Glazko, G.V.; Beggs, M.; Raj, V.; Davidson, M.K.; Wahls, W.P. A stress-activated, p38 mitogen-activated protein kinase-ATF/CREB pathway regulates posttranscriptional, sequence-dependent decay of target RNAs. Mol. Cell Biol. 2013, 33, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.S.; Wipf, P. Is Cdc25 a druggable target? Anticancer Agents Med. Chem. 2008, 8, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Lee, Y.M.; Nam, T.J.; Ko, Y.S.; Mah, S.; Kim, J.; Kim, Y.; Reddy, R.H.; Kim, Y.J.; Hong, S.; et al. Fascaplysin Exerts Anti-Cancer Effects through the Downregulation of Survivin and HIF-1α and Inhibition of VEGFR2 and TRKA. Int. J. Mol. Sci. 2017, 18, E2074. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Guru, S.K.; Manda, S.; Kumar, A.; Mintoo, M.J.; Prasad, V.D.; Sharma, P.R.; Mondhe, D.M.; Bharate, S.B.; Bhushan, S. A marine sponge alkaloid derivative 4-chloro fascaplysin inhibits tumor growth and VEGF mediated angiogenesis by disrupting PI3K/Akt/mTOR signaling cascade. Chem. Biol. Interact. 2017, 275, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Lee, J.H.; Kim, S.; Nam, T.J.; Kim, Y.S.; Kim, B.M.; Yim, W.J.; Lim, J.H. Fascaplysin Sensitizes Anti-Cancer Effects of Drugs Targeting AKT and AMPK. Molecules 2017, 23, E42. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, E310. [Google Scholar] [CrossRef] [PubMed]
- Mahale, S.; Bharate, S.B.; Manda, S.; Joshi, P.; Jenkins, P.R.; Vishwakarma, R.A.; Chaudhuri, B. Antitumour potential of BPT: A dual inhibitor of cdk4 and tubulin polymerization. Cell Death Dis. 2015, 6, e1743. [Google Scholar] [CrossRef] [PubMed]
- Lyakhova, I.A.; Bryukhovetsky, I.S.; Kudryavtsev, I.V.; Khotimchenko, Y.S.; Zhidkov, M.E.; Kantemirov, A.V. Antitumor Activity of Fascaplysin Derivatives on Glioblastoma Model In Vitro. Bull. Exp. Biol. Med. 2018, 164, 666–672. [Google Scholar] [CrossRef] [PubMed]


| CTC Cell Line | Fascaplysin | Cisplatin | ||
|---|---|---|---|---|
| Mean Ratio (TS/SC) | SD | Mean Ratio (TS/SC) | SD | |
| BHGc7 | 1.83 | 0.1 | 4.31 | 0.2 |
| BHGc10 | 1.63 | 0.2 | 2.32 | 0.4 |
| BHGc16 | 1.06 | 0.1 | 7.22 | 0.3 |
| BHGc26 | 0.77 | 0.1 | 5.20 | 0.3 |
| BHGc27 | 1.39 | 0.5 | 2.17 | 0.2 |
| UHGc5 | 0.99 | 0.1 | 4.8 | 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rath, B.; Hochmair, M.; Plangger, A.; Hamilton, G. Anticancer Activity of Fascaplysin against Lung Cancer Cell and Small Cell Lung Cancer Circulating Tumor Cell Lines. Mar. Drugs 2018, 16, 383. https://doi.org/10.3390/md16100383
Rath B, Hochmair M, Plangger A, Hamilton G. Anticancer Activity of Fascaplysin against Lung Cancer Cell and Small Cell Lung Cancer Circulating Tumor Cell Lines. Marine Drugs. 2018; 16(10):383. https://doi.org/10.3390/md16100383
Chicago/Turabian StyleRath, Barbara, Maximilian Hochmair, Adelina Plangger, and Gerhard Hamilton. 2018. "Anticancer Activity of Fascaplysin against Lung Cancer Cell and Small Cell Lung Cancer Circulating Tumor Cell Lines" Marine Drugs 16, no. 10: 383. https://doi.org/10.3390/md16100383




