Role of Mitochondria in Regulating Lutein and Chlorophyll Biosynthesis in Chlorella pyrenoidosa under Heterotrophic Conditions
Abstract
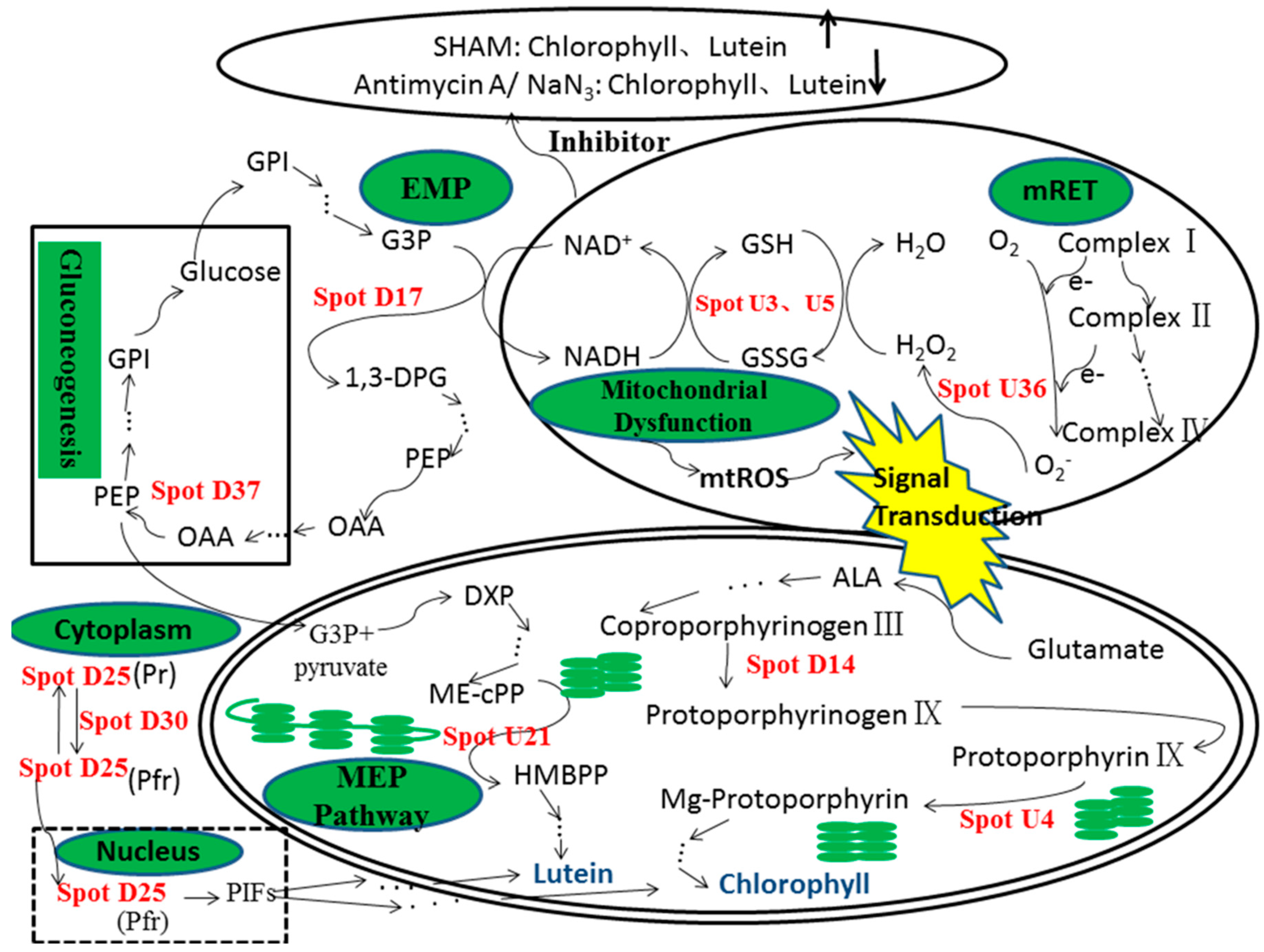
:1. Introduction
2. Results
2.1. Variation in Lutein and Chlorophyll Biosynthesis
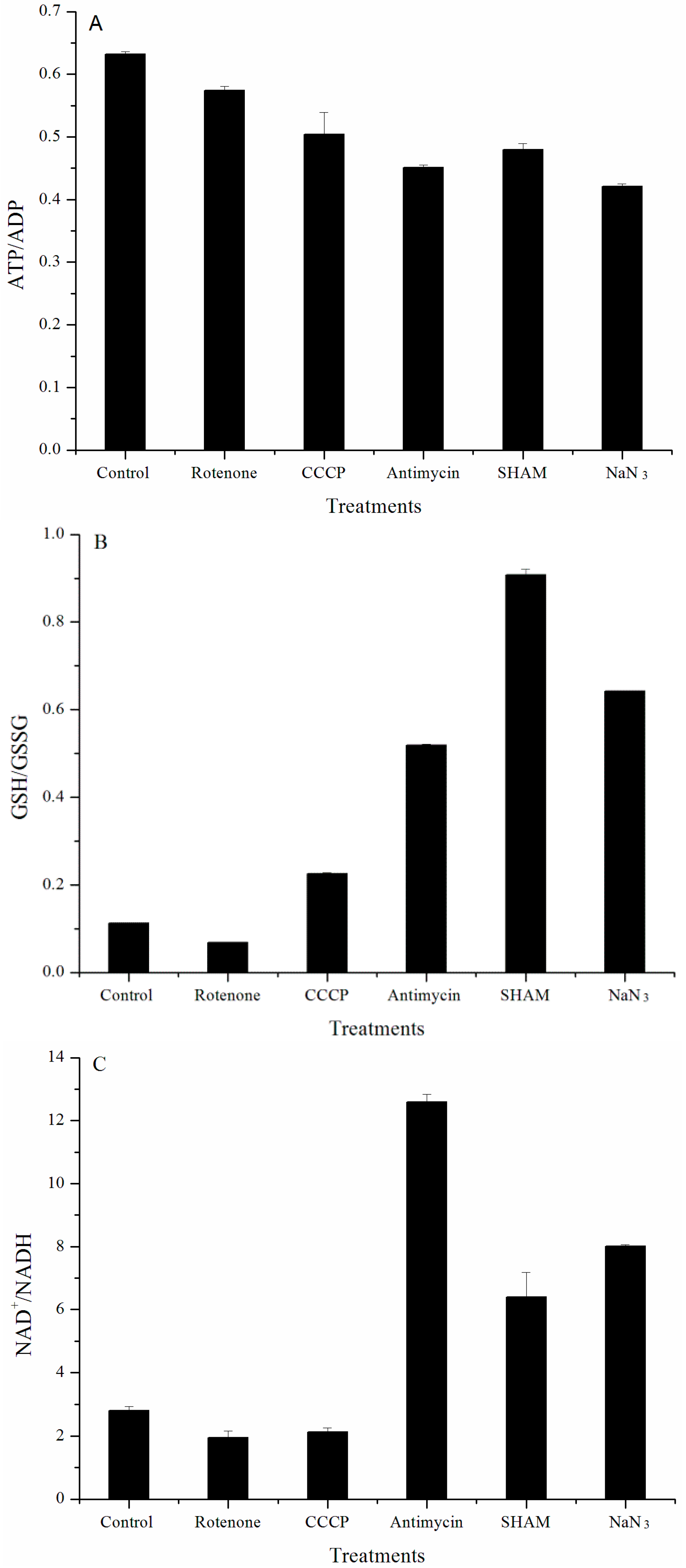
2.2. Analysis of Redox and Energy States
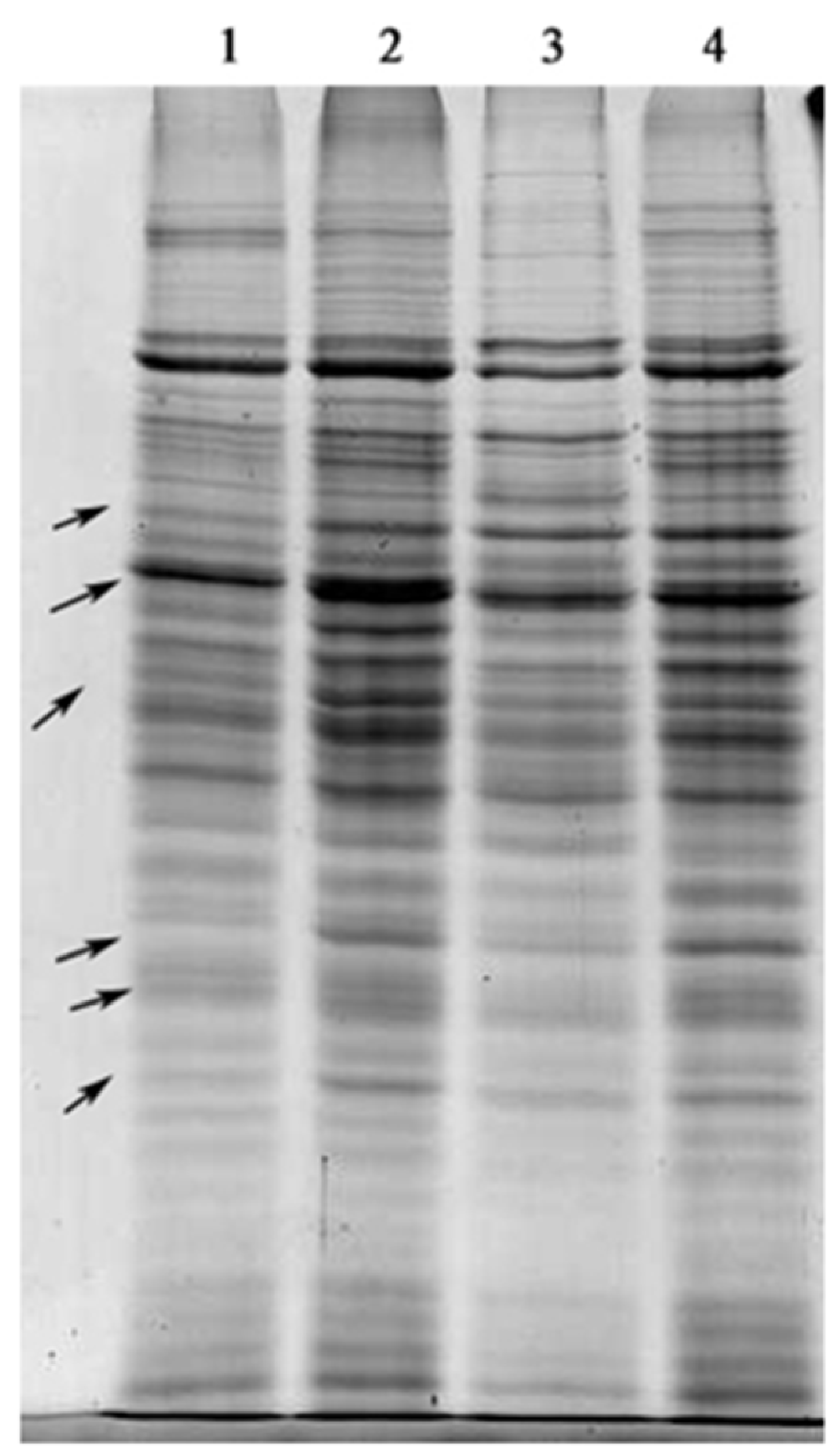
2.3. SDS-PAGE of Total Proteins
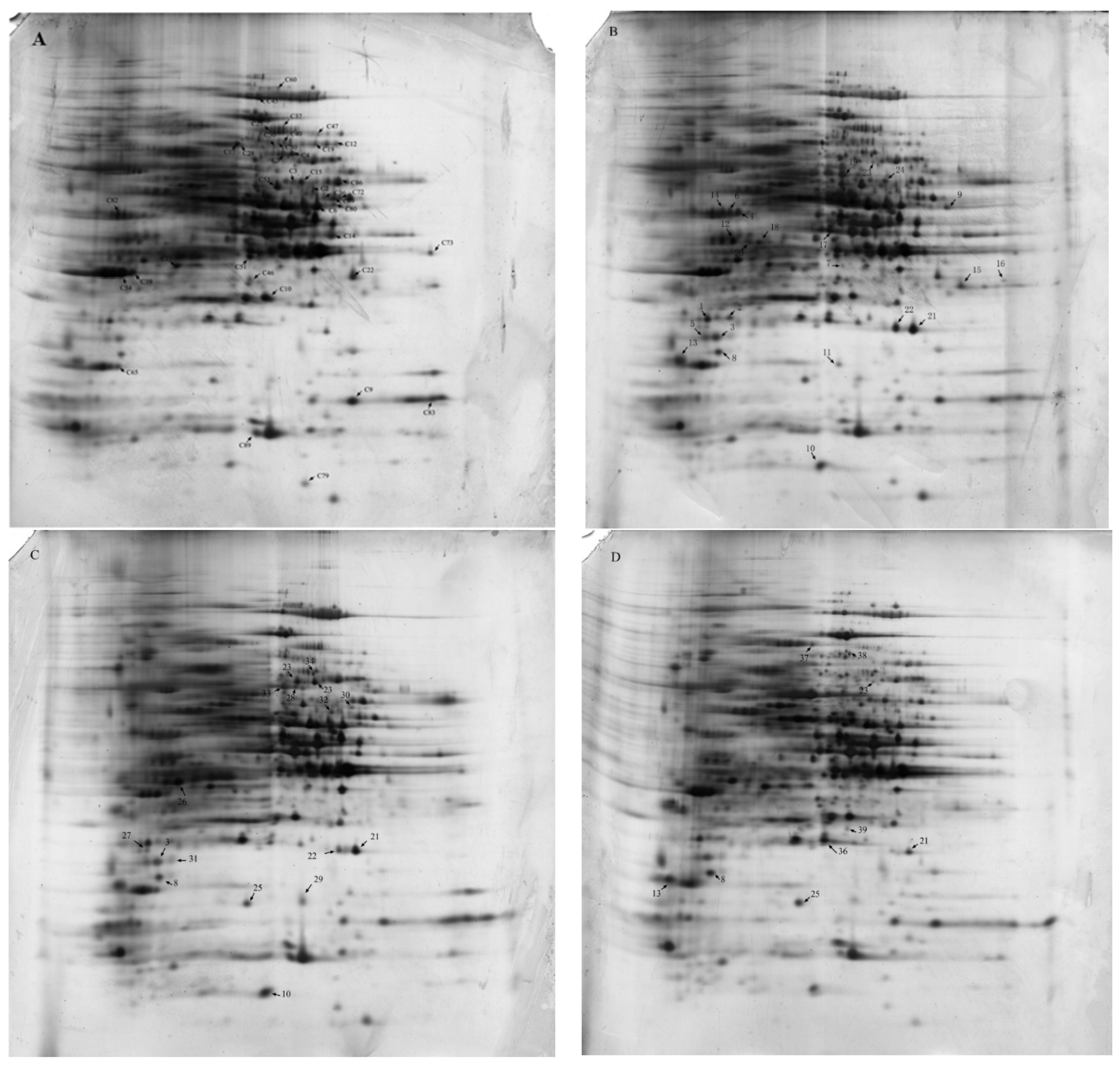
2.4. 2-DE Analysis and Identification of Differentially Expressed Proteins
2.5. Functional Categorization
3. Discussion
3.1. Up-Regulation of Antioxidant Proteins
3.2. Variation in Chloroplast Protein Expression Following Mitochondrial Dysfunction
3.3. Variation in Transcription and Protein Fate-Related Protein Expression
3.4. Variation in Metabolism and Energy-Related Protein Expression
4. Materials and Methods
4.1. Microalga and Culture Conditions
4.2. Mitochondrial Dysfunction
4.3. Pigment Analysis
4.4. Redox and Energy State Evaluation
4.5. Protein Extraction
4.6. One-Dimensional SDS Gel Electrophoresis and 2-DE
4.7. Gel Silver-Staining and Image Analysis
4.8. In-Gel Trypsin Digestion, Mass Spectrometry, and Protein Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, F. High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol. 1996, 14, 421–426. [Google Scholar] [CrossRef]
- Lee, Y.K. Commercial production of microalgae in the Asia-pacific rim. J. Appl. Phycol. 1997, 9, 403–411. [Google Scholar] [CrossRef]
- Qian, F.H. Study on the Optimization and Primary Scale-up of Chlorella Sequential Heterotrophic/Autotrophic Cultivation Technology for High-Density and High-Quality. Master’s Thesis, East China University of Science and Technology, Shanghai, China, 2007. [Google Scholar]
- Shi, X.M.; Chen, F.; Yuan, J.P.; Chen, H. Heterotrophic production of lutein by selected Chlorella strains. J. Appl. Phycol. 1997, 5, 445–450. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Qu, C.B.; Shi, X.M. Biochemical system analysis of lutein production by heterotrophic Chlorella pyrenoidosa in a fermentor. Food Technol. Biotechnol. 2009, 47, 450–455. [Google Scholar]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2015, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Le, M.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12. [Google Scholar]
- Sangiovanni, J.P.; Neuringer, M. The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: Promise of molecular genetics for guiding mechanistic and translational research in the field. Am. J. Clin. Nutr. 2012, 96, 1223S–1233S. [Google Scholar] [CrossRef] [PubMed]
- Mewborn, C.M.; Lindbergh, C.A.; Robinson, T.L.; Gogniat, M.A.; Terry, D.P.; Jean, K.R.; Hammond, B.R.; Renzi-Hammond, L.M.; Miller, L.S. Lutein and Zeaxanthin Are Positively Associated with Visual-Spatial Functioning in Older Adults: An fMRI Study. Nutrients 2018, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, M.; Yu, J. Mitochondrial retrograde regulation tuning fork in nuclear genes expressions of higher plants. J. Genet. Genom. 2008, 35, 65–71. [Google Scholar] [CrossRef]
- Matsuo, M.; Obokata, J. Remote control of photosynthetic genes by the mitochondrial respiratory chain. Plant J. 2006, 47, 873–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Huang, J.; Sandmann, G.; Chen, F. Glucose sensing and the mitochondrial alternative pathway are involved in the regulation of astaxanthin biosynthesis in the dark-grown Chlorella zofingiensis (chlorophyceae). Planta 2008, 228, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Padmasree, K.; Raghavendra, A. Response of photosynthetic carbon assimilation in mesophyll protoplasts to restriction on mitochondrial oxidative metabolism: Metabolites related to the redox status and sucrose biosynthesis. Photosynth. Res. 1999, 62, 231–239. [Google Scholar] [CrossRef]
- Saisho, D.; Nakazono, M.; Tsutsumi, N.; Hirai, A. ATP synthesis inhibitors as well as respiratory inhibitors increase steady-state level of alternative oxidase mRNA in Arabidopsis thaliana. J. Plant Physiol. 2001, 158, 241–245. [Google Scholar] [CrossRef]
- Li, N. Mitochondrial Complex I Inhibitor Rotenone Induces Apoptosis through Enhancing Mitochondrial Reactive Oxygen Species Production. J. Biol. Chem. 2002, 278, 8516–8525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhoads, D.M.; Vanlerberghe, G.C. Plant Mitochondria: From Genome to Function; Springer Dordrecht: Dordrecht, The Netherlands, 2004; pp. 83–106. ISBN 978-1-4020-2400-9. [Google Scholar]
- Tang, X.Q.; Luo, Y.X.; Chen, H.Z.; Liu, D.P. Mitochondria, endothelial cell function, and vascular diseases. Front. Physiol. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, D.P.; Nickels, R.; McIntosh, L. Evidence of mitochondrial involvement in the transduction of signals required for the induction of genes associated with pathogen attack and senescence. Plant J. 2002, 29, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, V.; Simonian, R.; Skulachev, V.; Starkov, A. Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria. FEBS Lett. 1997, 415, 87–90. [Google Scholar] [CrossRef]
- Umbach, A.L.; Fiorani, F.; Siedow, J.N. Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol. 2005, 139, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Claiborne, A.; Yeh, J.I.; Mallett, T.C.; Luba, J.; Crane, E.J.; Charrier, V.; Parsonage, D. Protein-sulfenic acids: Diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry 1999, 38, 15407–15416. [Google Scholar] [CrossRef] [PubMed]
- Grene, R. The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2002. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell Online 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Butow, R.A.; Avadhani, N.G. Mitochondrial signaling: The retrograde response. Mol. Cell 2004, 14, 1–15. [Google Scholar] [CrossRef]
- Yao, N.; Tada, Y.; Sakamoto, M.; Nakayashiki, H.; Park, P.; Tosa, Y.; Mayama, S. Mitochondrial oxidative burst involved in apoptotic response in oats. Plant J. 2002, 30, 567–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, H.Y.; Lin, Y.T.; Lin, G.; Wu, P.R.; Cheng, M.L. Nicotinamide nucleotide transhydrogenase (NNT) deficiency dysregulates mitochondrial retrograde signaling and impedes proliferation. Redox Biol. 2017, 12, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.; Maxwell, D.; Villarimo, A.; McIntosh, L. Mitochondria/nuclear signaling of alternative oxidase gene expression occurs through distinct pathways involving organic acids and reactive oxygen species. Plant Cell Rep. 2004, 23, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Beligni, M.V.; Prieto, S.; Haynes, P.A.; McDonald, W.H.; Yates, J.R.; Mayfield, S.P. Proteomic Characterization of the Chlamydomonas reinhardtii Chloroplast Ribosome identification of proteins unique to the 70s ribosome. J. Biol. Chem. 2003, 278, 33774–33785. [Google Scholar] [CrossRef] [PubMed]
- Rand, K.; Noll, C.; Schiebel, H.M.; Kemken, D.; Dülcks, T.; Kalesse, M.; Heinz, D.W.; Layer, G. The oxygen-independent coproporphyrinogen III oxidase HemN utilizes harderoporphyrinogen as a reaction intermediate during conversion of coproporphyrinogen III to protoporphyrinogen IX. Biol. Chem. 2010, 391, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Surpin, M.; Larkin, R.M.; Chory, J. Signal transduction between the chloroplast and the nucleus. Plant Cell Online 2002, 14, S327–S338. [Google Scholar] [CrossRef]
- Johanningmeier, U.; Howell, S.H. Regulation of light-harvesting chlorophyll-binding protein mRNA accumulation in Chlamydomonas reinhardi. Possible involvement of chlorophyll synthesis precursors. J. Biol. Chem. 1984, 259, 13541–13549. [Google Scholar] [PubMed]
- Oster, U.; Brunner, H.; Rüdiger, W. The greening process in cress seedlings. V. Possible interference of chlorophyll precursors, accumulated after thujaplicin treatment, with light-regulated expression of Lhc genes. J. Photochem. Photobiol. Biol. 1996, 36, 255–261. [Google Scholar] [CrossRef]
- Vinti, G.; Hills, A.; Campbell, S.; Bowyer, J.R.; Mochizuki, N.; Chory, J.; López-Juez, E. Interactions between hy1 and gun mutants of Arabidopsis, and their implications for plastid/nuclear signalling. Plant J. 2000, 24, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Zavgorodnyaya, A.; Papenbrock, J.; Grimm, B. Yeast 5-aminolevulinate synthase provides additional chlorophyll precursor in transgenic tobacco. Plant J. 1997, 12, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochizuki, N.; Brusslan, J.A.; Larkin, R.; Nagatani, A.; Chory, J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl. Acad. Sci. USA 2001, 98, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.J.; Willows, R.D. Mechanism and regulation of Mg-chelatase. Biochem. J. 1997, 327, 321–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kropat, J.; Oster, U.; Rüdiger, W.; Beck, C.F. Chloroplast signalling in the light induction of nuclear HSP70 genes requires the accumulation of chlorophyll precursors and their accessibility to cytoplasm/nucleus. Plant J. 2000, 24, 523–531. [Google Scholar] [CrossRef] [PubMed]
- La, R.N.; Rascio, N.; Oster, U.; Rüdiger, W. Amitrole treatment of etiolated barley seedlings leads to deregulation of tetrapyrrole synthesis and to reduced expression of Lhc and RbcS genes. Planta 2001, 213, 101–108. [Google Scholar]
- Moller, S.G.; Kunkel, T.; Chua, N.H. A plastidic ABC protein involved in intercompartmental communication of light signaling. Sci. Signal. 2001, 15, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Estevez, J.M.; Cantero, A.; Reindl, A.; Reichler, S.; Leon, P. 1-deoxy-d-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J. Biol. Chem. 2001, 276, 22901–22909. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.L.; Ducreux, L.J.; Hedden, P.; Millam, S.; Taylor, M.A. Overexpression of a bacterial 1-deoxy-d-xylulose 5-phosphate synthase gene in potato tubers perturbs the isoprenoid metabolic network: Implications for the control of the tuber life cycle. J. Exp. Bot. 2006, 57, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.W. Phytochromes are Pr-ipatetic kinases. Curr. Opin. Plant Biol. 1999, 2, 393–397. [Google Scholar] [CrossRef]
- Kuno, N.; Furuya, M. Phytochrome regulation of nuclear gene expression in plants. Semin. Cell Dev. Biol. 2000, 11, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Lintig, J.; Welsch, R.; Bonk, M.; Giuliano, G.; Batschauer, A.; Kleinig, H. Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. Plant J. 1997, 12, 625–634. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Qu, L.; Hager, J.; Chen, Z.; Zhao, H.; Deng, X.W. Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell Online 2001, 13, 2589–2607. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.H.; Jiang, S.C.; Lu, K.; Lu, Y.F.; Feng, X.J.; Wu, Z.; Liang, S.; Yu, Y.T.; Wang, X.F.; et al. Light-harvesting chlorophyll a/b-binding proteins, positively involved in abscisic acid signalling, require a transcription repressor, wrky40, to balance their function. J. Exp. Bot. 2013, 64, 5443–5456. [Google Scholar] [CrossRef] [PubMed]
- Toledoortiz, G.; Huq, E.; Rodríguez, C.M. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626–11631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Zhu, L.; Castillon, A.; Majee, M.; Downie, B.; Huq, E. Light-induced phosphorylation and degradation of the negative regulator phytochrome-interacting factor 1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell Online 2008, 20, 1586–1602. [Google Scholar] [CrossRef] [PubMed]
- Leivar, P.; Monte, E. Pifs: Systems integrators in plant development. Plant Cell 2014, 26, 56–78. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Park, J.; Kim, J.; Nagatani, A.; Lagarias, J.C.; Choi, G. Phytochrome B inhibits binding of phytochrome-interacting factors to their target promoters. Plant J. Cell Mol. Biol. 2012, 72, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, N.; Dolan, L. Origin and Diversification of Basic-Helix-Loop-Helix Proteins in Plants. Mol. Biol. Evol. 2010, 27, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Mummert, E.; Grimm, R.; Speth, V.; Eckerskorn, C.; Schiltz, E.; Gatenby, A.A.; Schäfer, E. ATCPl-related molecular chaperone from plants refolds phytochrome to its photoreversible form. Nature 1993, 363, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.S.; Lagarias, J.C. Light-independent phytochrome signaling mediated by dominant GAF domain tyrosine mutants of Arabidopsis phytochromes in transgenic plants. Plant Cell Online 2007, 19, 2124–2139. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, J.D.; Folta, K.M.; Paul, A.L.; Ferl, R.J. The 14-3-3 proteins µ and v influence transition to flowering and early phytochrome response. Plant Physiol. 2007, 145, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Emi, T.; Tominaga, M.; Sakamoto, K.; Shigenaga, A.; Doi, M.; Shimazaki, K.I. Blue-light-and phosphorylation-dependent binding of a 14-3-3 protein to phototropins in stomatal guard cells of broad bean. Plant Physiol. 2003, 133, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.P.; Adams, E.; Yanagawa, Y.; Matsui, M.; Shin, R. AtSKIP18 and AtSKIP31, F-box subunits of the SCF E3 ubiquitin ligase complex, mediate the degradation of 14-3-3 proteins in Arabidopsis. Biochem. Biophys. Res. Commun. 2017, 485, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Zhao, H.; Huang, F.; Zhang, Z.; Lin, W. The important functionality of 14-3-3 isoforms in rice roots revealed by affinity chromatography. J. Proteom. 2017, 158, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, X.; Guo, C.; Li, K. Spinach 14-3-3 protein interacts with the plasma membrane H(+)-ATPase and nitrate reductase in response to excess nitrate stress. Plant Physiol. Biochem. 2016, 106, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, L.T.; Sudom, A.M.; Prasad, L.; Leduc, Y.; Goldie, H. Structure/function studies of phosphoryl transfer by phosphoenolpyruvate carboxykinase. Biochim. Biophys. Acta-Proteins Proteom. 2004, 1697, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Streatfield, S.J.; Weber, A.; Kinsman, E.A.; Häusler, R.E.; Li, J.; Post, B.D.; Kaiser, W.M.; Pyke, K.A.; Flügge, U.I.; Chory, J. The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. Plant Cell Online 1999, 11, 1609–1621. [Google Scholar] [CrossRef]
- Cunningham, F.X. Regulation of carotenoid synthesis and accumulation in plants. Pure Appl. Chem. 2002, 74, 1409–1417. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Ye, J.; Zhang, J.; Lin, Y.; He, M.; Huang, J. Transcriptome analysis of chlorella zofingiensis to identify genes and their expressions involved in astaxanthin and triacylglycerol biosynthesis. Algal Res. 2016, 17, 236–243. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, L.; Yu, M.; An, X.; Ma, R.; Yu, Z. Proteomic analysis of changes in mitochondrial protein expression during peach fruit ripening and senescence. J. Proteom. 2016, 147, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Baroli, I. Zeaxanthin Accumulation in the Absence of a Functional Xanthophyll Cycle Protects Chlamydomonas reinhardtii from Photooxidative Stress. Plant Cell Online 2003, 15, 992–1008. [Google Scholar] [CrossRef]
- Lazzarino, G.; Amorini, A.M.; Fazzina, G.; Vagnozzi, R.; Signoretti, S.; Donzelli, S.; Stasio, E.D.; Giardina, B.; Tavazzi, B. Single-sample preparation for simultaneous cellular redox and energy state determination. Anal. Biochem. 2003, 322, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ganzera, M.; Vrabl, P.; Wörle, E.; Burgstaller, W.; Stuppner, H. Determination of adenine and pyridine nucleotides in glucose-limited chemostat cultures of Penicillium simplicissimum by one-step ethanol extraction and ion-pairing liquid chromatography. Anal. Biochem. 2006, 359, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Wang, S.B.; Hu, Q.; Sommerfeld, M.; Chen, F. An optimized protocol for isolation of soluble proteins from microalgae for two-dimensional gel electrophoresis analysis. J. Appl. Phycol. 2003, 15, 485–496. [Google Scholar] [CrossRef]
- Wessel, D.; Flügge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, Y.; Cheung, Y.H.; Yang, Z.; Chiu, J.F.; Che, C.M.; He, Q.Y. Proteomic approach to study the cytotoxicity of dioscin (saponin). Proteomics 2006, 6, 2422–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, F.; Lu, X.P.; Zeng, H.L.; He, Q.Y.; Xiong, S.; Jin, L.; He, Q.Y. Proteomic and functional analyses reveal a dual molecular mechanism underlying arsenic-induced apoptosis in human multiple myeloma cells. J. Proteome Res. 2009, 6, 3006–3019. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Etten, J.L.V. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 2010, 22, 2943–2955. [Google Scholar] [CrossRef] [PubMed]
- Waridel, P.; Frank, A.; Thomas, H.; Surendranath, V.; Sunyaev, S.; Pevzner, P.; Shevchenko, A. Sequence similarity-driven proteomics in organisms with unknown genomes by LC-MS/MS and automated de novo sequencing. Proteomics 2007, 14, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
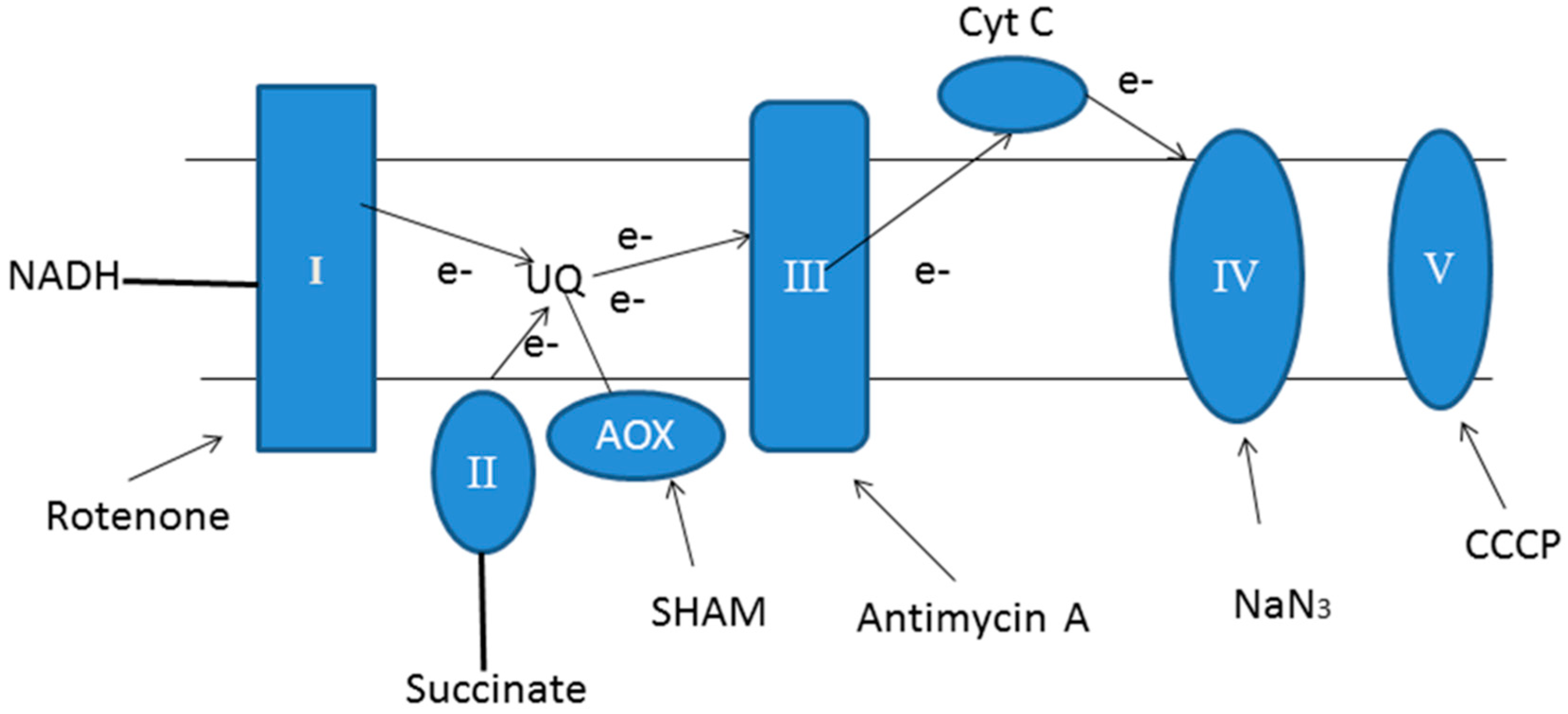
| Inhibitors | Biomass (%) | Lutein (%) | Chl. a (%) | Chl. b (%) |
|---|---|---|---|---|
| Antimycin A (0.1 mM) | 59.5 ± 3.2 | 87.7 ± 3.5 | 73.2 ± 2.3 | 73.4 ± 2.5 |
| NaN3 (0.05 mM) | 68.9 ± 9.6 | 49.1 ± 1.4 | 38.9 ± 0.9 | 45.2 ± 1.7 |
| SHAM (0.5 mM) | 46.1 ± 8.6 | 156.9 ± 7.2 | 121.6 ± 3.6 | 136.1 ± 5.4 |
| CCCP (0.032 mM) | 42.7 ± 2.6 | 106.4 ± 3.2 | 104.5 ± 1.8 | 128.6 ± 2.8 |
| Rotenone (0.032 mM) | 41.4 ± 3.1 | 107.1 ± 1.6 | 103.1 ± 1.0 | 104.9 ± 0.4 |
| Spot a | Protein Name b | Protein Function and Categorization c | Protein ID d | Species e | MW/pI | Peptides f | Score g |
|---|---|---|---|---|---|---|---|
| Down-regulated proteins | |||||||
| D2 | Autophagy-related protein 3 | Protein transport, Protein fate (folding, modification, destination) | gi|307105862 | Chlorella vulgaris | 35,252.9/4.42 | 1 | 35 |
| D3 | Adenylosuccinate synthetase | Purine nucleotide synthesis, Metabolism | gi|307108106 | Chlorella vulgaris | 53,409.7/6.62 | 3 | 64 |
| D4 | Hypothetical protein CHLNCDRAFT_144859 | GMP synthase, Metabolism | gi|307108123 | Chlorella vulgaris | 58,172.5/5.6 | 8 | 96 |
| D5 | Hypothetical protein CHLNCDRAFT_56182 | Adenylosuccinate synthetase, Metabolism | gi|307106668 | Chlorella vulgaris | 64,272.6/5.81 | 4 | 59 |
| D6 | Phosphoserine aminotransferase | L-glutamate synthesis, Metabolism | gi|307109471 | Chlorella vulgaris | 44,960.5/5.60 | 1 | 37 |
| D9 | Hypothetical protein CHLNCDRAFT_139931 | Alternative splicing factor SRp20/9G8, Transcription | gi|307103428 | Chlorella vulgaris | 19,849.2/11.52 | 6 | 53 |
| D10 | Hypothetical protein CHLNCDRAFT_134964 | SWI-SNF chromatin remodeling complex, Snf 5 subunit, Transcription | gi|307106629 | Chlorella vulgaris | 22,841.5/5.85 | 1 | 27 |
| D12 | Nitrite reductase | NO biosynthesis, Cell rescue, defense and virulence | gi|116265919 | Chlorella vulgaris | 22,954.7/9.11 | 1 | 60 |
| D14 | Coproporphyrinogen III oxidase | Key enzyme in heme synthesis, Metabolism | gi|71082810 | Candidatus Pelagibacter ubique | 32,383/9.83 | 1 | 68 |
| D15 | Hypothetical protein CHLNCDRAFT_30336 | ABC transporter superfamily, lipid transport, Protein with binding function or cofactor requirement | gi|307109169 | Chlorella vulgaris | 65,272.95/8.50 | 1 | 26 |
| D17 | Hypothetical protein CHLNCDRAFT_56437 | d-3-phosphoglycerate dehydrogenase, Metabolism | gi|307111670 | Chlorella vulgaris | 63,615.5/6.45 | 1 | 41 |
| D19 | Nitrite reductase | NO biosynthesis, Cell rescue, defense and virulence | gi|116265919 | Chlorella vulgaris | 22,954.7/9.11 | 1 | 69 |
| D21 | Malate dehydrogenase | Synthesis of oxaloacetate, Energy | gi|307103202 | Chlorella vulgaris | 35,063.6/8.2 | 6 | 104 |
| D22 | Hypothetical protein CHLNCDRAFT_57327 | Galactokinase activity, Protein with binding function or cofactor requirement | gi|307109337 | Chlorella vulgaris | 55,429.4/5.97 | 10 | 66 |
| D25 | Phytochrome A | G-protein coupled photoreceptor activity, Transcription | P42500 | Glycine max | 125,653.3/6.16 | 14 | 54 |
| D30 | Hypothetical protein CHLNCDRAFT_58635 | Chaperonin complex component, TCP-1 eta subunit (CCT7), Protein fate (folding, modification, destination) | gi|307105118 | Chlorella variabilis | 61,530.4/6.25 | 1 | 28 |
| D36 | Hypothetical protein CHLNCDRAFT_56384 | UDP-glucose 4-epimerase/UDP-sulfoquinovose synthase, Protein with binding function or cofactor requirement | gi|307103315 | Chlorella variabilis | 39,120.5/6.53 | 1 | 77 |
| D37 | Hypothetical protein CHLNCDRAFT_49080 | Phosphoenolpyruvate carboxykinase activity | gi|307104333 | Chlorella variabilis | 49,051.4/5.69 | 6 | 70 |
| D39 | Hypothetical protein CHLNCDRAFT_31785 | 14-3-3 family, multifunctional chaperone, Protein with binding function or cofactor requirement | gi|307106152 | Chlorella variabilis | 29,385.8/4.96 | 2 | 77 |
| D40 | Hypothetical protein CHLNCDRAFT_34933 | Prolyl-tRNA aminoacylation, Protein with binding function or cofactor requirement | gi|307109063 | Chlorella variabilis | 33,163.8/6.56 | 2 | 83 |
| D45 | Hypothetical protein CHLNCDRAFT_14282 | Aconitase/homoaconitase | gi|307110485 | Chlorella variabilis | 11,454.9/5.88 | 3 | 53 |
| D46 | Expressed protein | Unknown, Unknown | gi|307104059 | Chlorella variabilis | 19,035.7/7.02 | 1 | 28 |
| D50 | Aspartate carbamoyltransferase | Enzyme of the first committed step in pyrimidine synthesis, Protein activity regulation | P49077 | Arabidopsis thaliana | 43,139.3/6.21 | 8 | 55 |
| D51 | Hypothetical protein CHLNCDRAFT_35404 | Protein serine/threonine kinase, Protein fate (folding, modification, destination) | gi|307107220 | Chlorella variabilis | 40,979.4/9.01 | 6 | 56 |
| D53 | Hypothetical protein CHLNCDRAFT_52952 | Calcium ion binding, Protein with binding function or cofactor requirement | gi|307106250 | Chlorella variabilis | 245,122.6/7.28 | 1 | 27 |
| D54 | 14-3-3-like protein | Multifunctional chaperone, posttranslational modification, Protein with binding function or cofactor requirement | P52908 | Chlamydomonas reinhardtii | 29,495.8/4.9 | 1 | 32 |
| D60 | Elongation factor 2 (EF-2) | Catalyze GTP dependent ribosomal translocation step during translation elongation, Protein with binding function or cofactor requirement | gi|119167 | Parachlorella kessleri | 94,054.8/5.84 | 17 | 308 |
| D65 | Expressed protein | Splicing coactivator, RNA processing, Transcription | gi|307109910 | Chlorella variabilis | 84,395.1/10.28 | 1 | 28 |
| D69 | Citrate synthase | Citrate synthesis, located in mitochondria, Energy | gi|307105555 | Chlorella variabilis | 53,533.7/7.24 | 5 | 98 |
| D72 | Hypothetical protein CHLNCDRAFT_56456 | Dystonin, growth -arrest-specific protein, cytoskeletone, Subcellular localization | gi|307111694 | Chlorella variabilis | 311,562.1/4.77 | 8 | 66 |
| D73 | Ribosomal protein | Large ribosomal subunit, Protein synthesis | gi|307103203 | Chlorella variabilis | 25,401/8.65 | 10 | 138 |
| D80 | Hypothetical protein CHLNCDRAFT_138729 | Electron transport, Protein with binding function or cofactor requirement | gi|307104457 | Chlorella variabilis | 62,588.1/9.52 | 11 | 53 |
| D82 | OSJNBb0032E06.9 | Ribonuclease H activity, Cell cycle and DNA processing | gi|38344375 | Oryza stiva | 138,142.2/8.96 | 15 | 83 |
| D83 | Protein W01F3.3a (mlt-11) | Serine-type endopeptidase inhibitor activity, Development | gi|115534910 | Caenorhabditis elegans | 236,516.9/5.07 | 10 | 84 |
| D86 | Fumarate hydratase | Catalyze S-malate synthesis, mitochondria | gi|17549498 | Ralstonia solanacearum GMI1000 | 49,372.3/6.07 | 4 | 127 |
| D89 | Ribulose bisphosphate carboxylase small chain 1 | Carbon dioxide fixation, Energy | P00873 | Chlamydomonas reinhardtii | 20,606.4/9.36 | 2 | 66 |
| Up-regulated proteins | |||||||
| U1 | Chloroplast thioredoxin peroxidase | Peroxidase activity, Cell rescue, defense and virulence | gi|294845922 | Volvox carteri f. nagariensis | 17,421/5.15 | 2 | 193 |
| U2 | SMC domain protein | Chromosome structure maintenance, Unknown | gi|296427824 | Heliothis subflexa | 65,238/5.63 | 87 | |
| U3 | Peroxiredoxin TSA1 | Oxidoreductase, cell redox homeostasis, Cell rescue, defense and virulence | gi|126132194 | Scheffersomyces stipitis CBS 6054 | 21,761/4.92 | 2 | 82 |
| U4 | Magnesium chelatase subunit of protochlorophyllide reductase | Chlorophyll biosynthesis, Protein with binding function or cofactor requirement | gi|254798626 | Parachlorella kessleri | 39,567/5.08 | 6 | 310 |
| U5 | 2-Cys peroxiredoxin | Oxidoreductase, cell redox homeostasis, Cell rescue, defense and virulence | gi|327506370 | Dunaliella viridis | 22,235/5.74 | 1 | 51 |
| U6 | Hypothetical protein CHLNCDRAFT_48133 | Ornithine carbamoyltransferase, Protein with binding function or cofactor requirement | gi|307109894 | Chlorella variabilis | 38,764.5/6.68 | 9 | 51 |
| U7 | Hypothetical protein CHLNCDRAFT_53139 | Antioxidant activity, Cell rescue, defense and virulence | gi|307106076 | Chlorella variabilis | 21,778.9/8.35 | 1 | 40 |
| U8 | Hypothetical protein BAL199_15803 | Unknown, Unknown | gi|163792326 | alpha proteobacterium | 42,102.3/5.49 | 12 | 88 |
| U9 | Hypothetical protein CHLNCDRAFT_48477 | Membrane transport, Cellular transport, transport facilitation and transport routes | gi|307110872 | Chlorella vulgaris | 31,637.3/9.49 | 1 | 29 |
| U10 | tRNA(Ile)-lysidine synthase, chloroplastic | Ligase activity, translation, Transcription | Q32RX0 | Staurastrum punctulatum | 58,797/10.16 | 1 | 29 |
| U11 | Penecillin-binding protein 2 | Penicillin binding, Protein with binding function or cofactor requirement | gi|163752395 | Shewanella benthica | 68,820.33/9.54 | 67 | |
| U12 | Aldehyde dehydrogenase | Oxidation of aldehyde, Metabolism | gi|285018869 | Xanthomonas albilineans | 54,004.7/6.05 | 64 | |
| U13 | Hypothetical protein CHLNCDRAFT_30965 | Structural constitute of ribosome, Protein synthesis | gi|307107744 | Chlorella vulgaris | 21,281.2/10.33 | 8 | 51 |
| U14 | Hypothetical protein CHLNCDRAFT_143237 | Dystonin, growth arrest specific protein, Subcelluar location | gi|307109339 | Chlorella vulgaris | 56,981.7/5.56 | 13 | 53 |
| U15 | FG-GAP repeat protein | Ligand binding, Unknown | gi|40062562 | Uncultureed marine bacterium 159 | 136,477.9/4.18 | 66 | |
| U16 | Expressed protein | Unknown, Unknown | gi|307111048 | Chlorella variabilis | 20,888.8/10.28 | 6 | 57 |
| U17 | Hypothetical protein CHLNCDRAFT_59525 | nuclear receptor binding factor-1, Cell rescue, defense and virulence | gi|307111650 | Chlorella variabilis | 34,897.1/5.06 | 68 | |
| U18 | Hypothetical protein PEPMIC_01485 | Unknown, Unknown | gi|160947550 | Peptostreptococcus micros | 18,170.3/4.67 | 7 | 83 |
| U19 | Hypothetical protein CHLNCDRAFT_58231 | Ribosomal protein L22, Protein synthesis | gi|307105888 | Chlorella vulgaris | 67,650.2/10.05 | 1 | 29 |
| U20 | C protein alpha-antigen | Receptor, Protein with binding function or cofactor requirement | gi|307708369 | Streptococcus mitis NCTC 1226 | 34,6246.9/4.98 | 65 | |
| U21 | 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase | Oxidoreductase, terpenoids biosynthesis, Protein with binding function or cofactor requirement | Q5QYA9 | Idiomarina loihiensis | 40,462/5.68 | 1 | 58 |
| U22 | N-(5’-phosphoribosyl)anthranilate isomerase | Tryptophan biosynthesis, Metabolism | gi|307107003 | Chlorella vulgaris | 20,906.8/5.55 | 1 | 31 |
| U23 | Hypothetical protein CHLNCDRAFT_37743 | Aldehyde dehydrogenase, Metabolism | gi|307102335 | Chlorella variabilis | 45,285.3/6.11 | 3 | 111 |
| U24 | Hypothetical protein OsI_036678 | Calcium ion binding, Protein with binding function or cofactor requirement | gi|125536231 | Oryza sativa | 31,743.5/9.98 | 10 | 93 |
| U25 | Chloroplast 30S ribosomal protein S4 | Structural constituent of ribosome, Protein synthesis | P59137 | Catharomnion ciliatum | 23,589.9/10.3 | 1 | 30 |
| U26 | Hypothetical protein CHLNCDRAFT_140182 | Transcription initiation factor, Transcription | gi|307103188 | Chlorella variabilis | 63,894.8/5.19 | 13 | 54 |
| U27 | Nitrate reductase [NADH] 1 | Catalyze nitrite synthesis, Metabolism | P16081 | Oryza sativa | 101,447.9/6.19 | 1 | 29 |
| U28 | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit | Carbon fixation, Energy | gi|164455027 | Chlorella variabilis | 52,496.3/5.99 | 1 | 32 |
| U29 | Hypothetical protein MGG_08723 | Unknown, Unknown | gi|145601241 | Magnaporthe grisea | 36,967.8/5.69 | 8 | 83 |
| U30 | Expressed protein | Large subunit of ribosome, Protein synthesis | gi|307108236 | Chlorella variabilis | 9805.4/11.71 | 4 | 69 |
| U31 | Hypothetical protein CHLNCDRAFT_59740 | 3-oxoacyl-(acyl-carrier-protein) synthase, lipid transport, Cellular transport, transport facilitation and transport routes | gi|307104988 | Chlorella variabilis | 32,230.3/6.19 | 1 | 28 |
| U32 | Hypothetical protein CHLNCDRAFT_18194 | Unknown, Unknown | gi|307111928 | Chlorella variabilis | 9084.4/4.37 | 1 | 28 |
| U33 | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit | Carbon fixation, Energy | gi|164455037 | Chlorella variabilis | 52,496.3/6.0 | 18 | 193 |
| U34 | Glucose-6-phosphate isomerase | Isomerase, involved in glycolysis, Energy | gi|284434863 | Parachlorella kessleri | 27,035/5.35 | 1 | 48 |
| U35 | Hypothetical protein CHLNCDRAFT_143799 | Unknown, Unknown | gi|307108818 | Chlorella variabilis | 43,154.4/9.57 | 1 | 27 |
| U36 | Superoxide dismutase | Antioxidant enzyme, Cellular communication/signal transduction mechanism | gi|34558145 | Wolinella succinogenes DSM 1740 | 25,795.1/8.89 | 78 | |
| U37 | Hypothetical protein CHLNCDRAFT_138879 | FAP-dependent helicase activity, Transcription | gi|307104244 | Chlorella variabilis | 59,382.6/9.77 | 1 | 27 |
| U38 | Glucose -6-phosphate isomerase | Glycolysis enzyme, Energy | gi|307105594 | Chlorella variabilis | 72,048.5/6.41 | 7 | 53 |
| U39 | GTP-binding protein | Intracellular protein transport, Cellular communication/signal transduction mechanism | gi|307106020 | Chlorella variabilis | 25,468.9/6.66 | 2 | 66 |
| Spot a | F.D. h | ||
|---|---|---|---|
| SHAM | Antimycin A | NaN3 | |
| Down-regulated proteins | |||
| D2 | −4.95 ± 0.58 | −5.27 ± 2.16 | |
| D3 | −6.57 ± 0.59 | ||
| D4 | −3.01 ± 0.11 | −2.37 ± 0.22 | −2.29 ± 0.86 |
| D5 | −2.22 ± 0.30 | −1.93 ± 0.20 | |
| D6 | −2.18 ± 0.11 | ||
| D9 | −1.81 ± 0.35 | ||
| D10 | −1.77 ± 0.35 | ||
| D12 | −2.37 ± 0.74 | −3.12 ± 0.05 | |
| D14 | −1.95 ± 0.20 | −1.98 ± 0.43 | −2.29 ± 0.59 |
| D15 | −2.27 ± 0.66 | −6.95 ± 2.48 | |
| D17 | −2.45 ± 0.98 | −4.46 ± 1.59 | −3.87 ± 0.79 |
| D19 | −2.42 ± 0.98 | −2.08 ± 0.25 | |
| D21 | −2.26 ± 0.82 | ||
| D22 | −1.87 ± 0.26 | −6.32 ± 1.36 | −1.87 ± 0.26 |
| D25 | >−100 | >−100 | |
| D30 | >−100 | >−100 | |
| D36 | −3.03 ± 1.22 | −3.08 ± 0.91 | |
| D37 | −3.32 ± 0.65 | −5.66 ± 0.04 | |
| D39 | −2.27 ± 0.57 | ||
| D40 | >−100 | >−100 | |
| D45 | −2.03 ± 0.02 | ||
| D46 | −1.77 ± 0.37 | ||
| D50 | −1.98 ± 0.14 | ||
| D51 | −1.65 ± 0.24 | ||
| D53 | −1.89 ± 0.46 | −1.89 ± 0.46 | |
| D54 | −1.85 ± 0.33 | ||
| D60 | >−100 | ||
| D65 | >−100 | ||
| D69 | −3.55 ± 0.81 | ||
| D72 | −2.54 ± 0.97 | ||
| D73 | −4.20 ± 1.37 | ||
| D80 | −2.24 ± 0.52 | ||
| D82 | −1.89 ± 0.25 | ||
| D83 | −1.89 ± 0.30 | ||
| D86 | −1.59 ± 0.20 | ||
| D89 | −1.76 ± 0.38 | ||
| Up-regulated proteins | |||
| U1 | 1.65 ± 0.57 | ||
| U2 | 1.72 ± 0.48 | ||
| U3 | 1.53 ± 0.16 | 1.62 ± 0.08 | |
| U4 | 1.60 ± 0.09 | ||
| U5 | 1.84 ± 0.37 | ||
| U6 | 1.92 ± 0.46 | ||
| U7 | 1.85 ± 0.33 | ||
| U8 | 1.65 ± 0.57 | 1.59 ± 0.28 | 1.94 ± 0.68 |
| U9 | 1.54 ± 0.18 | ||
| U10 | 2.13 ± 0.37 | 2.75 ± 0.02 | |
| U11 | 1.68 ± 0.30 | ||
| U12 | 2.53 ± 0.78 | ||
| U13 | 2.56 ± 0.73 | 3.96 ± 2.05 | |
| U14 | 2.18 ± 0.10 | ||
| U15 | 3.08 ± 1.38 | ||
| U16 | 2.66 ± 0.45 | ||
| U17 | 2.21 ± 0.25 | ||
| U18 | 3.46 ± 0.93 | ||
| U19 | 4.38 ± 1.02 | ||
| U20 | 4.05 ± 0.09 | ||
| U21 | 5.36 ± 0.05 | 3.75 ± 0.01 | 1.53 ± 0.21 |
| U22 | 6.89 ± 1.10 | 3.61 ± 0.86 | |
| U23 | >100.00 | 5.39 ± 0.69 | |
| U24 | >100.00 | ||
| U25 | 1.79 ± 0.53 | 1.89 ± 0.39 | |
| U26 | 1.77 ± 0.39 | ||
| U27 | 1.60 ± 0.05 | ||
| U28 | 1.92 ± 0.48 | ||
| U29 | 1.92 ± 0.44 | ||
| U30 | 2.36 ± 0.71 | ||
| U31 | 2.56 ± 0.75 | ||
| U32 | 2.16 ± 1.08 | ||
| U33 | 3.02 ± 0.08 | ||
| U34 | >100 | ||
| U35 | 1.75 ± 0.21 | ||
| U36 | 1.60 ± 0.03 | ||
| U37 | 2.33 ± 0.20 | ||
| U38 | >100 | ||
| U39 | 3.75 ± 0.01 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-h.; Li, T.; He, Q.-y.; Sun, Z.; Jiang, Y. Role of Mitochondria in Regulating Lutein and Chlorophyll Biosynthesis in Chlorella pyrenoidosa under Heterotrophic Conditions. Mar. Drugs 2018, 16, 354. https://doi.org/10.3390/md16100354
Liu Z-h, Li T, He Q-y, Sun Z, Jiang Y. Role of Mitochondria in Regulating Lutein and Chlorophyll Biosynthesis in Chlorella pyrenoidosa under Heterotrophic Conditions. Marine Drugs. 2018; 16(10):354. https://doi.org/10.3390/md16100354
Chicago/Turabian StyleLiu, Zhi-hui, Tao Li, Qing-yu He, Zheng Sun, and Yue Jiang. 2018. "Role of Mitochondria in Regulating Lutein and Chlorophyll Biosynthesis in Chlorella pyrenoidosa under Heterotrophic Conditions" Marine Drugs 16, no. 10: 354. https://doi.org/10.3390/md16100354





