Nine New Triterpene Glycosides, Magnumosides A1–A4, B1, B2, C1, C2 and C4, from the Vietnamese Sea Cucumber Neothyonidium (=Massinium) magnum: Structures and Activities against Tumor Cells Independently and in Synergy with Radioactive Irradiation
Abstract
:1. Introduction
2. Results and Discussion
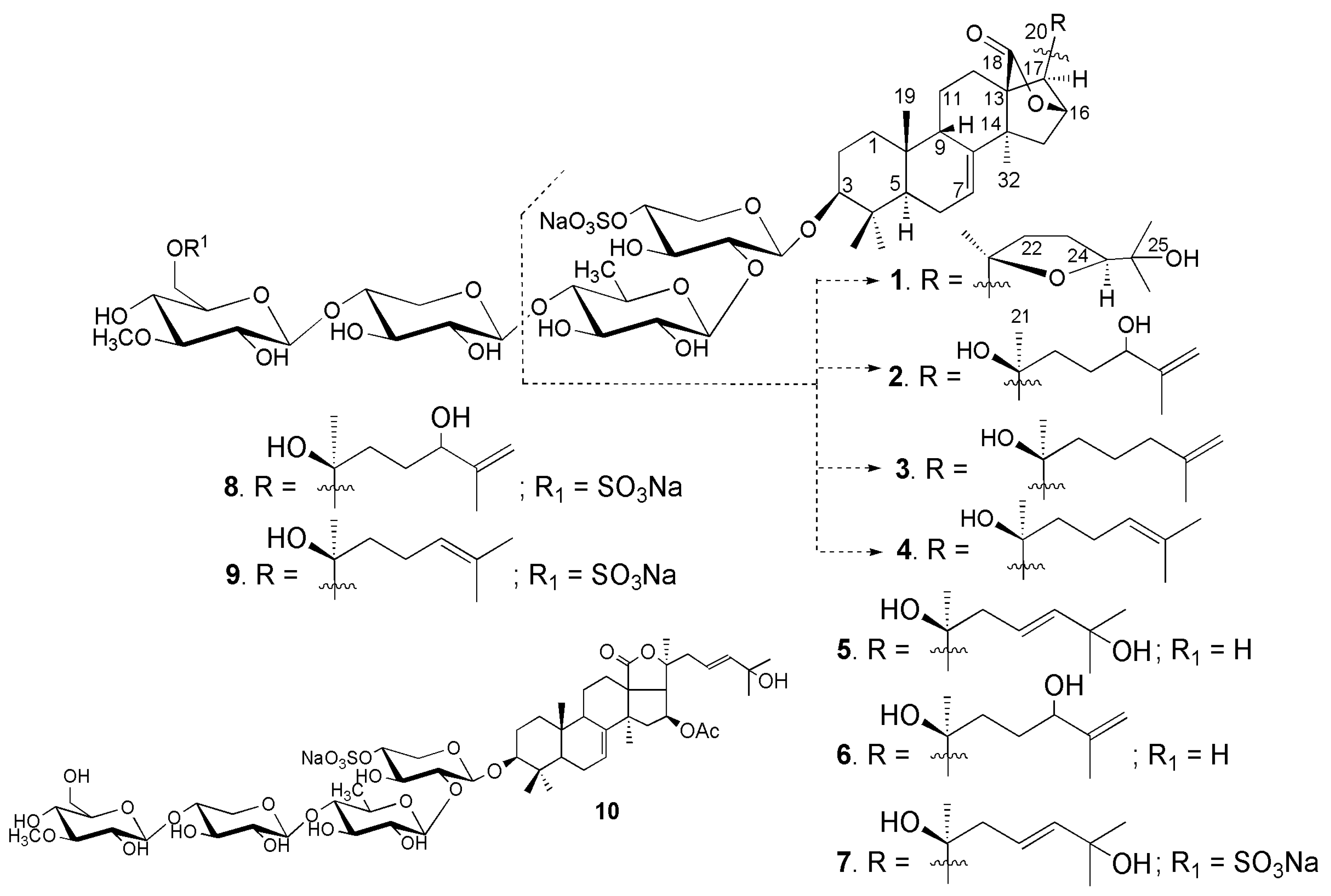
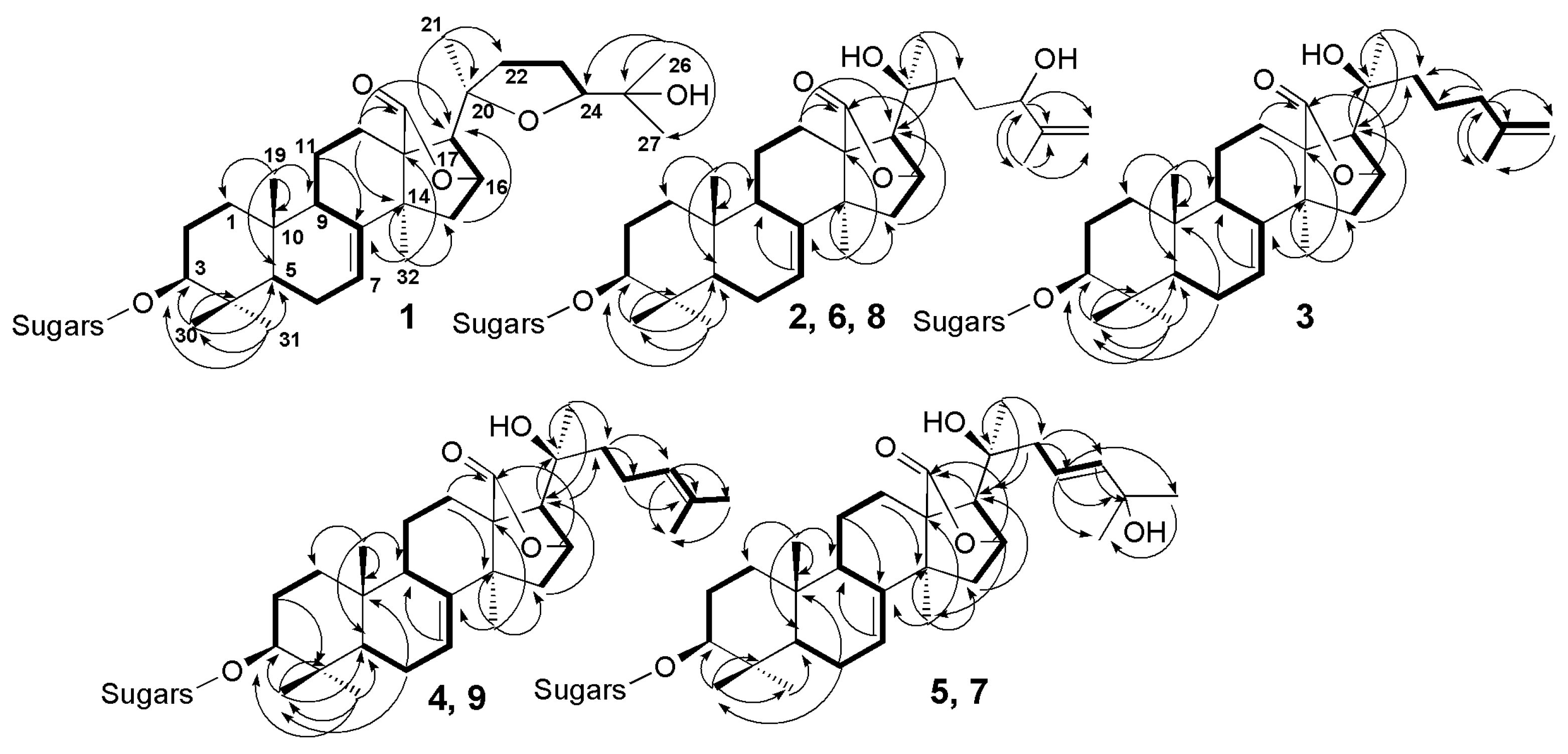
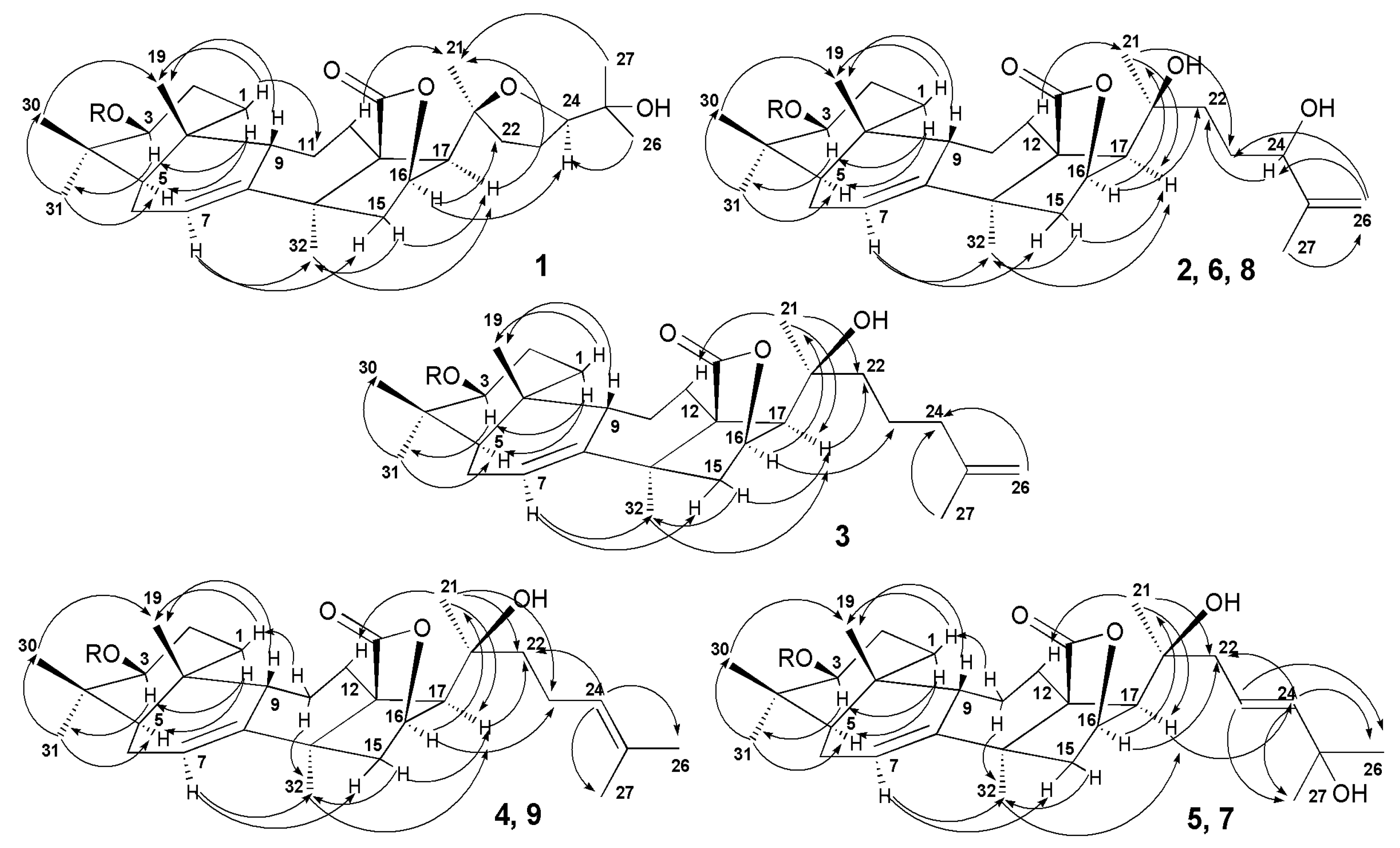
2.1. Structural Elucidation of the Glycosides
2.2. Biological Activities of Glycosides
2.2.1. Hemolytic and Cytotoxic Activities of the Glycosides 1–9 against Mouse Spleenocites and the Ascites Form of Mouse Ehrlich Carcinoma Cells
2.2.2. The Effect of the Glycosides on Cell Viability of Human Colorectal Adenocarcinoma DLD-1 Cells
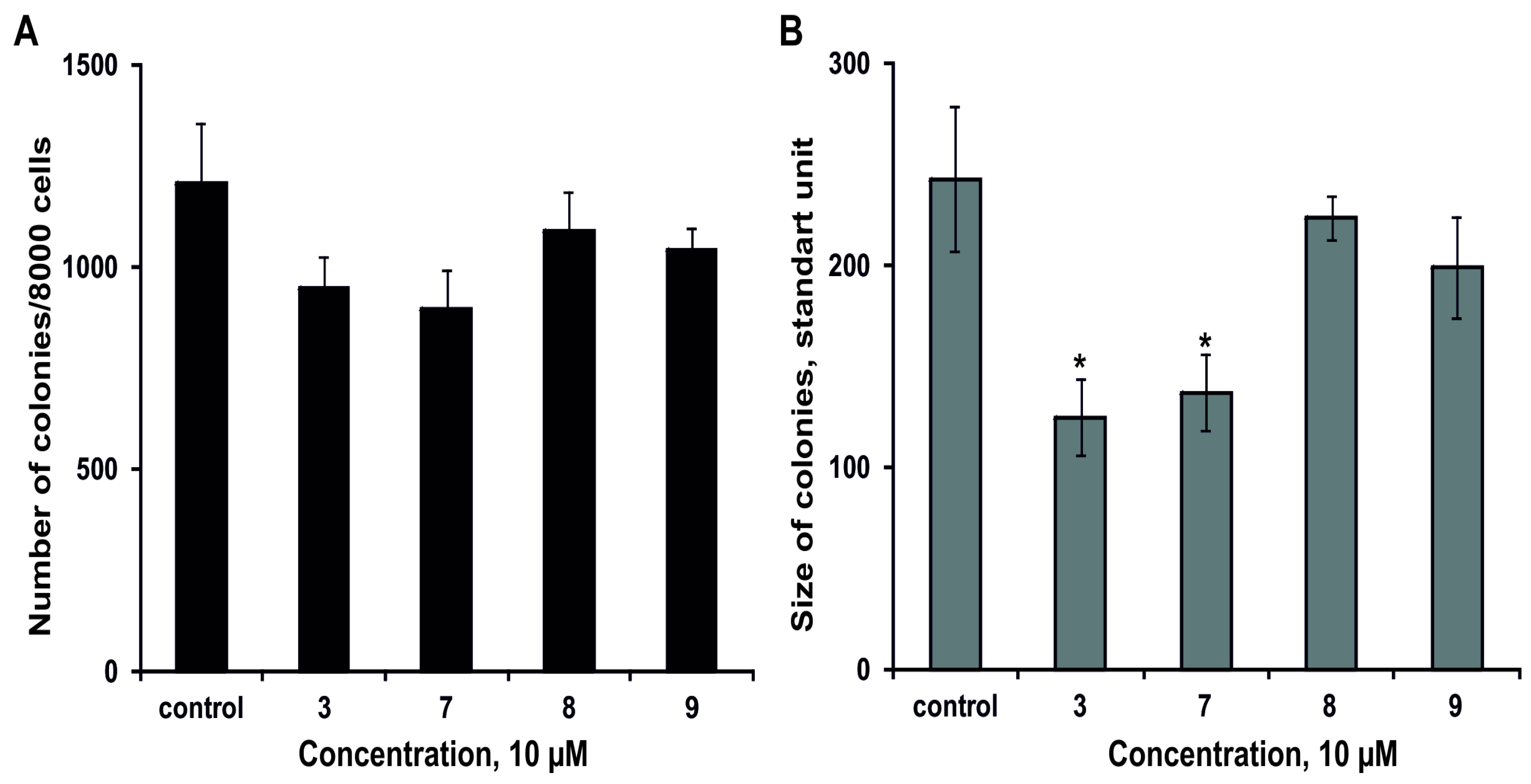
2.2.3. The Effect of the Glycosides on Formation and Growth of Colonies of Human Colorectal Adenocarcinoma DLD-1 Cells
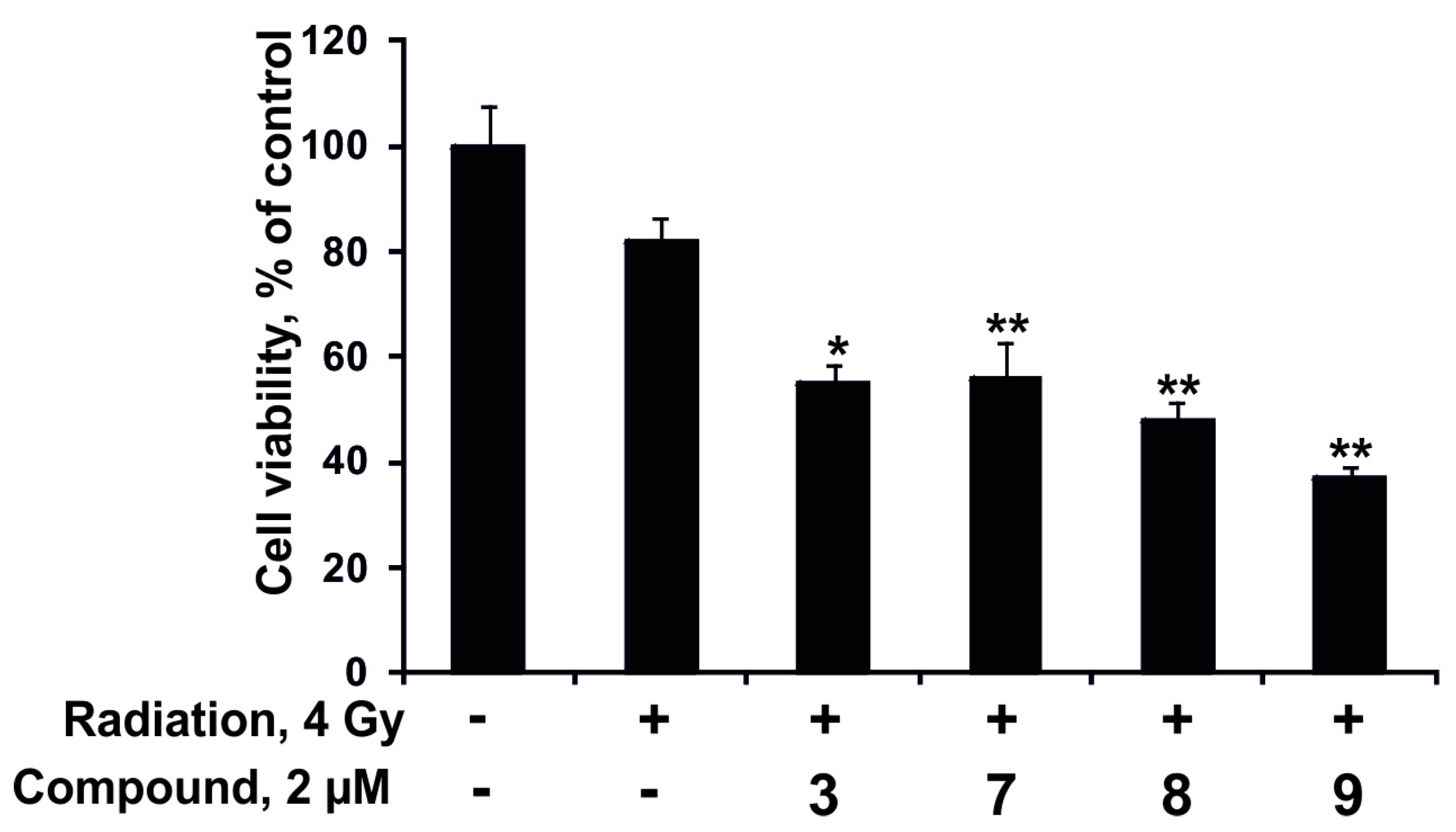
2.2.4. The Synergism of Radioactive Irradiation and the Compounds Effects on Proliferation and Colony Formation of Human Colorectal Adenocarcinoma Cells
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.3.1. Magnumoside A1 (1)
3.3.2. Magnumoside A2 (2)
3.3.3. Magnumoside A3 (3)
3.3.4. Magnumoside A4 (4)
3.3.5. Magnumoside B1 (5)
3.3.6. Magnumoside B2 (6)
3.3.7. Magnumoside C1 (7)
3.3.8. Magnumoside C2 (8)
3.3.9. Magnumoside C4 (9)
3.4. Preparation of the MTPA Esters of Compound 2
3.5. Bioassay
3.5.1. Cell Culture
3.5.2. Cytotoxic Activity
3.5.3. Hemolytic Activity
3.5.4. DLD-1 Human Colorectal Adenocarcinoma Cell Culture
3.5.5. Cytotoxicity against DLD-1 Cells Assay
3.5.6. Soft Agar Assay
3.5.7. DLD-1 Cell Proliferation Assay
3.5.8. Radiation Exposure
3.5.9. Cell Irradiation
3.5.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stonik, V.A.; Kalinin, V.I.; Avilov, S.A. Toxins from the sea cucumbers (Holothuroids): Chemical structures, properties, taxonomic distribution, biosynthesis and evolution. J. Nat. Toxins 1999, 8, 235–248. [Google Scholar] [PubMed]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Smirnov, A.V. Sea cucumbers triterpene glycosides, the recent progress in structural elucidation and chemotaxonomy. Phytochem. Rev. 2005, 4, 221–236. [Google Scholar] [CrossRef]
- Bahrami, Y.; Franko, C.M.M. Acetylated triterpene glycosides and their biological activity from Holothurioidea reported in the past six decades. Mar. Drugs 2016, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides of sea cucumbers (Holothuroidea, Echinodermata) as taxonomic markers. Nat. Prod. Commun. 2015, 10, 21–26. [Google Scholar] [PubMed]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A. Taxonomic significance and ecological role of triterpene glycosides from Holothurians. Biol. Bull. 2016, 43, 532–540. [Google Scholar] [CrossRef]
- Honey-Escandon, M.; Arreguin-Espinosa, R.; Solis-Martin, F.A.; Samyn, Y. Biological and taxonomic perspective of triterpenoid glycosides of sea cucumbers of the family Holothuriidae (Echinodermata, Holothuroidea). Comp. Biochem. Physiol. 2015, 180B, 16–39. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides from sea cucucmbers (Holothurioidae, Echinodermata), biological activities and functions. In Studies in Natural Product Chemistry (Bioactive Natural Products); Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2008; Volume 35, pp. 135–196. [Google Scholar]
- Kim, S.K.; Himaya, S.W.A. Triterpene glycosides from sea cucucmbers and their biological activities. Adv. Food Nutr. Res. 2012, 63, 297–319. [Google Scholar]
- Aminin, D.L.; Pislyagin, E.A.; Menchinskaya, E.S.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Immunomodulatory and anticancer activity of sea cucumber triterpene glycosides. In Studies in Natural Products Chemistry (Bioactive Natural Products); Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2014; Volume 41, pp. 75–94. [Google Scholar]
- Careaga, V.P.; Maier, M.S. Cytotoxic triterpene glycosides from sea cucumbers. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 515–528. [Google Scholar]
- Aminin, D.L.; Menchinskaya, E.S.; Pislyagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer activity of sea cucumber triterpene glycosides. Mar. Drugs 2015, 13, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Mohammed, A.; Rao, C. Sea cucumbers metabolites as potent anti-cancer agents. Mar. Drugs 2015, 13, 2909–2923. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Dyshlovoy, S.A.; Kuzmich, A.S.; Shubina, L.K.; Avilov, S.A.; Silchenko, A.S.; Bode, A.M.; Dong, Z.; Stonik, V.A. In vitro anticancer activities of some triterpene glycosides from holothurians of Cucumariidae, Stichopodidae, Psolidae, Holothuriidae and Synaptidae families. Nat. Prod. Commun. 2016, 11, 1239–1242. [Google Scholar]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Sea cucumber triterpene glycosides as anticancer agents. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2016; Volume 49, pp. 55–105. [Google Scholar]
- Zurita, M.B.; Ahond, A.; Poupat, C.; Potier, P. Invertebres marins du lagon Neo-Caledonien, VII. Etude structurale d’un nouveau saponoside sulfate extrait de l’holothurie Neothyonidium magnum. J. Nat. Prod. 1986, 49, 809–813. [Google Scholar] [CrossRef]
- Avilov, S.A.; Kalinovskii, A.I.; Stonik, V.A. New triterpene glycoside from the holothurian Neothyonidium magnum. Chem. Nat. Comp. 1990, 26, 42–45. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Yurchenko, E.A.; Dolmatov, I.Y. Colochirosides B1, B2, B3 and C, novel sulfated triterpene glycosides from the sea cucumber Colochirus robustus (Cucumariidae, Dendrochirotida). Nat. Prod. Commun. 2015, 10, 1687–1694. [Google Scholar] [PubMed]
- Silchenko, A.S.; Stonik, V.A.; Avilov, S.A.; Kalinin, V.I.; Kalinovsky, A.I.; Zaharenko, A.M.; Smirnov, A.V.; Mollo, E.; Cimino, G. Holothurins B2, B3 and B4, New triterpene glycosides from Mediteranean sea cucumbers of the genus Holothuria. J. Nat. Prod. 2005, 68, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Li, L.; Sun, P.; Yi, Y.H. Lecanorosides A and B, two new triterpene glycosides from the sea cucumber Actinopyga lecanora. J. Asian Nat. Prod. Res. 2008, 10, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhang, W.; Liu, B.-S.; Pan, M.-X.; Wang, X.-H. A novel sulfated holostane glycoside from sea cucumber Holothuria leucospilota. Chem. Biodivers. 2010, 7, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Kalinovskii, A.I.; Stonik, V.A. Onekotanogenin—A new triterpene genin from the holothurian Psolus fabricii. Chem. Nat. Comp. 1987, 23, 560–563. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Dolmatov, I.Y.; Dautov, S.S.; Stonik, V.A.; Kalinin, V.I. Colochiroside E, an usual non-holostane triterpene sulfated trioside from the sea cucumber Colochirus robustus and evidence of the impossibility of a 7(8)-double bond migration in lanostane derivatives having an 18(16)-lactone. Nat. Prod. Commun. 2016, 11, 741–746. [Google Scholar] [PubMed]
- Ilyin, S.G.; Reshetnyak, M.V.; Afiyatullov, S.S.; Stonik, V.A.; Elyakov, G.B. The crystal and molecular structure of diacetate of holost-8(9)-en-3α,16β-diol. Rep. USSR Acad. Sci. 1985, 284, 356–359. [Google Scholar]
- Ilyin, S.G.; Sharypov, V.F.; Stonik, V.A.; Antipin, M.Y.; Struchkov, Y.T.; Elyakov, G.B. The crystal and molecular structure of (23S)-acetoxy-9β-holost-7-en-3β-ol and stereochemical peculiarities of the double bond migration from 7(8) to 8(9) and 9(11)-positions in holostane-type triterpenoids. Bioorg. Chem. 1991, 17, 1123–1128. [Google Scholar]
- Kalinovsky, A.I.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. The assignment of the absolute configuration of C-22 chiral center in the aglycones of triterpene glycosides from the sea cucumber Cladolabes schmeltzii and chemical transformations of cladoloside C. Nat. Prod. Commun. 2015, 10, 1167–1170. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Yurchenko, E.A.; Dolmatov, I.Y. Colochirosides A1, A2, A3 and D, four novel sulfated triterpene glycosides from the sea cucumber Colochirus robustus (Cucumariidae, Dendrochirotida). Nat. Prod. Commun. 2016, 11, 381–387. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Yurchenko, E.A.; Dautov, S.S. Structures of violaceusosides C, D, E and G, sulfated triterpene glycosides from the sea cucumber Pseudocolochirus violaceus (Cucumariidae, Denrochirotida). Nat. Prod. Commun. 2014, 9, 391–399. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I.; Jayasandhya, P.; Rajan, G.C.; Padmakumar, K.P. Structures and biological activities of typicosides A1, A2, B1, C1 and C2, triterpene glycosides from the sea cucumbers Actinocucumis typica. Nat. Prod. Commun. 2013, 8, 301–310. [Google Scholar] [PubMed]
- Zou, Z.R.; Yi, Y.H.; Xu, Q.Z.; Wu, H.M.; Lin, H.W. A new disulfated triterpene glycoside from the sea cucumber Mensamaria intercedens Lampert. Chin. Chem. Lett. 2003, 14, 585–587. [Google Scholar]
- Zhang, S.-Y.; Yi, Y.-H.; Tang, H.-F. Cytotoxic sulfated triterpene glycosides from the sea cucmber Pseudocolochirus violaceus. Chem. Biodivers. 2006, 3, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Avilov, S.A.; Kalinin, V.I.; Makarieva, T.N.; Stonik, V.A.; Kalinovskii, A.I. Structure of Cucumarioside G2, a novel nonholostane glycoside from the sea cucumber Eupentacta fraudatrix. J. Nat. Prod. 1994, 57, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Kalinin, V.I. Structures and cytotoxic properties of cucumariosides H2, H3 and H4 from the sea cucumber Eupentacta fraudatrix. Nat. Prod. Res. 2012, 26, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and cytotoxic action of cucumariosides A2, A7, A9, A10, A11, A13 and A14, seven new minor non-sulated tetraosides and an aglycone with an uncommon 18-hydroxy group. Nat. Prod. Commun. 2012, 7, 845–852. [Google Scholar] [PubMed]
- Avilov, S.A.; Antonov, A.S.; Drozdova, O.A.; Kalinin, V.I.; Kalinovsky, A.I.; Stonik, V.A.; Riguera, R.; Lenis, L.A.; Jimenez, C. Triterpene glycosides from the Far-Eastern sea cucumber Pentamera calcigera. 1. Monosulfated glycosides and cytotoxicity of their unsulfated derivatives. J. Nat. Prod. 2000, 63, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Kalinivskii, A.I.; Stonik, V.A. Psolusoside A—The main triterpene glycoside from the holothurian Psolus fabricii. Chem. Nat. Compd. 1985, 21, 197–202. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Kalinovskii, A.I.; Stonik, V.A.; Dmitrenok, P.S.; El’kin, Y.N. Structure of psolusoside B—A nonholostane triterpene glycoside of the holothurian genus Psolus. Chem. Nat. Compd. 1989, 25, 311–317. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjashenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Stonik, V.A. 3β-O-Glycosylated 16β-acetoxy-9β-H-lanosta-7,24-diene-3β,18,20β-triol, an intermediate metabolite from the sea cucumber Eupentacta fraudatrix and its biosynthetic significance. Biochem. Syst. Ecol. 2012, 44, 53–60. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Song, Y.M.; Wang, B.; Zhang, F.R.; Yang, R.; Wang, H.Q.; Zhang, G.J. Ginsenoside Rg3 sensitizes human non-small cell lung cancer cells to γ-radiation by targeting the nuclear factor-κB pathway. Mol. Med. Rep. 2015, 12, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The fucoidans from brown algae of Far-Eastern seas: Anti-tumor activity and structure-function relationship. Food Chem. 2013, 141, 1211–1217. [Google Scholar] [CrossRef] [PubMed]


| Position | 1–4, δC a | 1–4, δH b | 5, 6, δC a | 5, 6, δH b | 7–9, δC a | 7–9, δH b |
|---|---|---|---|---|---|---|
| Xyl (1→C-3) | ||||||
| 1 | 104.8 CH | 4.66 (d, 7.0) | 104.8 CH | 4.65 (d, 7.3) | 104.8 CH | 4.65 (d, 7.0) |
| 2 | 82.3 CH | 4.00 (t, 8.7) | 82.2 CH | 4.00 (t, 8.7) | 82.3 CH | 3.98 (t, 8.8) |
| 3 | 75.1 CH | 4.24 (t, 8.7) | 75.2 CH | 4.25 (t, 8.7) | 75.0 CH | 4.24 (t, 8.8) |
| 4 | 76.1 CH | 5.01 m | 76.0 CH | 5.04 m | 76.1 CH | 4.99 m |
| 5 | 63.9 CH2 | 4.77 (dd, 5.2; 11.7) | 63.9 CH2 | 4.77 (dd, 5.5; 11.9) | 63.9 CH2 | 4.76 (dd, 5.4; 11.5) |
| 3.72 (t, 11.7) | 3.71 (dd, 9.6; 11.9) | 3.71 (t, 11.8) | ||||
| Qui (1→2Xyl) | ||||||
| 1 | 105.2 CH | 5.00 (d, 7.6) | 104.7 CH | 5.00 (d, 7.8) | 104.7 CH | 4.98 (d, 7.7) |
| 2 | 76.4 CH | 3.90 (t, 9.3) | 75.8 CH | 3.88 (t, 9.1) | 75.8 CH | 3.87 (t, 8.6) |
| 3 | 76.8 CH | 4.04 (t, 9.3) | 74.7 CH | 3.97 (t, 9.1) | 74.8 CH | 3.95 (t, 9.0) |
| 4 | 76.1 CH | 3.61 (t, 8.7) | 85.6 CH | 3.51 (t, 9.1) | 85.6 CH | 3.50 (t, 9.0) |
| 5 | 72.8 CH | 3.66 (dd, 5.8; 8.7) | 71.3 CH | 3.65 (dd, 5.9; 9.6) | 71.4 CH | 3.63 (dd, 6.1; 9.3) |
| 6 | 18.1 CH3 | 1.53 (d, 5.9) | 17.8 CH3 | 1.61 (d, 5.9) | 17.8 CH3 | 1.60 (d, 6.1) |
| Xyl (1→4Qui) | ||||||
| 1 | 104.4 CH | 4.77 (d, 7.7) | 104.3 CH | 4.76 (d, 7.7) | ||
| 2 | 73.3 CH | 3.89 (t, 8.6) | 73.1 CH | 3.85 (t, 8.6) | ||
| 3 | 86.4 CH | 4.12 (t, 8.6) | 87.0 CH | 4.04 (t, 8.8) | ||
| 4 | 68.7 CH | 3.94 m | 68.7 CH | 3.90 (t, 8.7) | ||
| 5 | 65.8 CH | 4.11 (dd, 5.6; 11.3) | 65.7 CH | 4.11 (dd, 5.3; 11.8) | ||
| 3.59 (t, 10.8) | 3.59 (t, 11.3) | |||||
| MeGlc (1→3Xyl) | ||||||
| 1 | 104.4 CH | 5.21 (d, 8.0) | 104.6 CH | 5.12 (d, 7.9) | ||
| 2 | 74.5 CH | 3.88 (t, 8.7) | 74.3 CH | 3.80 (t, 9.4) | ||
| 3 | 87.0 CH | 3.68 (t, 8.6) | 86.4 CH | 3.64 (t, 9.4) | ||
| 4 | 70.4 CH | 3.89 m | 69.9 CH | 3.96 (t, 9.4) | ||
| 5 | 77.5 CH | 3.90 m | 75.5 CH | 4.03 m | ||
| 6 | 61.7 CH2 | 4.38 (dd, 1.7; 11.5) | 67.1 CH2 | 4.97 (d, 9.4) | ||
| 4.05 (dd, 5.9; 11.5) | 4.72 (dd, 5.5; 11.0) | |||||
| OMe | 60.5 CH3 | 3.80 s | 60.5 CH3 | 3.76 s |
| Position | 1 a | 2, 6, 8 b | 3 b | 4, 9 a | 5, 7 b |
|---|---|---|---|---|---|
| 1 | 1.46 m | 1.35 m | 1.34 m | 1.46 m | 1.33 m |
| 2 | 2.10 m, Hα | 1.94 m, Hα | 1.94 m, Hα | 2.09 m, Hα | 1.93 m, Hα |
| 1.89 m, Hβ | 1.74 m, Hβ | 1.73 m, Hβ | 1.87 m, Hβ | 1.73 m, Hβ | |
| 3 | 3.26 (dd, 3.9, 11.6) | 3.16 (dd, 3.8, 11.5) | 3.16 (dd, 4.3, 11.7) | 3.26 (dd, 4.1, 11.8) | 3.16 (dd, 4.5, 11.4) |
| 5 | 0.98 (dd, 3.3; 11.6) | 0.86 (brd, 11.0) | 0.86 (dd, 3.9; 11.7) | 0.99 (dd, 3.6; 11.8) | 0.86 (dd, 3.3; 11.9) |
| 6 | 2.06 m, Hα | 1.92 m, Hα | 1.91 m, Hα | 2.05 m, Hα | 1.91 m, Hα |
| 1.96 m, Hβ | 1.82 m, Hβ | 1.81 m, Hβ | 1.97 m, Hβ | 1.81 m, Hβ | |
| 7 | 5.61 (dt, 2.3; 7.4) | 5.57 m | 5.59 (brd, 7.4) | 5.63 (dt, 2.4; 7.3) | 5.56 (brd, 7.5) |
| 9 | 3.18 (brd, 13.7) | 3.03 (brd, 15.2) | 3.02 (brd, 14.9) | 3.23 (brd, 13.5) | 3.02 (brd, 14.7) |
| 11 | 2.03 m, Hβ | 1.93 m | 1.94 m | 2.03 m, Hβ | 1.95 m |
| 1.51 m, Hα | 1.40 m | 1.41 m | 1.54 m, Hα | 1.42 m | |
| 12 | 2.47 m, Hβ | 2.50 m, Hβ | 2.50 m | 2.62 m, Hβ | 2.47 m, Hβ |
| 2.03 m, Hα | 2.14 m, Hα | 2.09 m | 2.14 m, Hα | 2.10 m, Hα | |
| 15 | 2.12 (d, 13.2, Hβ) | 2.08 (d, 13.5, Hβ) | 2.10 (d, 13.5, Hβ) | 2.15 (d, 13.3, Hβ) | 2.08 (d, 13.8, Hβ) |
| 1.89 (dd, 2.6; 13.5, Hα) | 2.01 (dd, 2.0; 13.3, Hα) | 2.05 (brd, 11.5, Hα) | 1.94 (dd, 2.6; 13.4, Hα) | 2.00 (dd, 2.6; 13.4, Hα) | |
| 16 | 4.84 br s | 5.12 br s | 5.08 br s | 5.05 (d, 1.6) | 5.14 br s |
| 17 | 2.48 s | 2.72 s | 2.69 s | 2.63 s | 2.77 s |
| 19 | 1.04 s | 0.91 s | 0.91 s | 1.05 s | 0.91 s |
| 21 | 1.32 s | 1.46 s | 1.44 s | 1.50 s | 1.47 s |
| 22 | 2.02 m | 2.05 m | 1.70 m | 1.83 m | 2.50 (br d, 6.9) |
| 1.73 m | 1.83 m | --- | 1.80 m | --- | |
| 23 | 2.08 m | 2.06 m | 1.72 m | 2.40 m | 5.92 (d, 15.8) |
| 1.87 m | 1.97 m | 1.62 m | 2.31 m | --- | |
| 24 | 3.97 (dd, 5.6; 9.3) | 4.33 (t, 6.0) | 2.00 (t, 7.4) | 5.27 (t, 7.1) | 6.13 (dt, 6.9; 8.0; 15.8) |
| 26 | 1.31 s | 5.16 br s | 4.74 br s | 1.70 s | 1.48 s |
| --- | 4.87 br s | 4.73 br s | --- | --- | |
| 27 | 1.42 s | 1.82 s | 1.65 s | 1.63 s | 1.48 s |
| 30 | 1.15 s | 0.97 s | 0.97 s | 1.15 s | 0.97 s |
| 31 | 1.31 s | 1.16 s | 1.16 s | 1.31 s | 1.16 s |
| 32 | 1.36 s | 1.37 s | 1.40 s | 1.41 s | 1.39 s |
| Position | 1 a | 2, 6, 8 b | 3 b | 4, 9 a | 5, 7 b |
|---|---|---|---|---|---|
| 1 | 35.8 CH2 | 35.6 CH2 | 35.6 CH2 | 35.8 CH2 | 35.6 CH2 |
| 2 | 26.9 CH2 | 26.7 CH2 | 26.7 CH2 | 26.9 CH2 | 26.7 CH2 |
| 3 | 88.8 CH | 88.9 CH | 89.0 CH | 88.8 CH | 89.0 CH |
| 4 | 39.4 C | 39.1 C | 39.2 C | 39.4 C | 39.2 C |
| 5 | 47.7 CH | 47.5 CH | 47.6 CH | 47.6 CH | 47.5 CH |
| 6 | 23.2 CH2 | 23.1 CH2 | 23.1 CH2 | 23.2 CH2 | 23.1 CH2 |
| 7 | 122.4 CH | 122.3 CH | 122.4 CH | 122.4 CH | 122.4 CH |
| 8 | 147.6 C | 147.6 C | 147.6 C | 147.8 C | 147.6 C |
| 9 | 46.1 CH | 45.9 CH | 46.0 CH | 46.0 CH | 45.9 CH |
| 10 | 35.5 C | 35.3 C | 35.4 C | 35.5 C | 35.4 C |
| 11 | 21.8 CH2 | 21.7 CH2 | 21.7 CH2 | 21.9 CH2 | 21.7 CH2 |
| 12 | 20.1 CH2 | 20.1 CH2 | 20.2 CH2 | 20.4 CH2 | 20.1 CH2 |
| 13 | 54.9 C | 54.6 C | 54.7 C | 54.6 C | 54.7 C |
| 14 | 45.6 C | 45.9 C | 46.0 C | 45.8 C | 46.0 C |
| 15 | 44.4 CH2 | 44.2 CH2 | 44.3 CH2 | 44.5 CH2 | 44.2 CH2 |
| 16 | 79.6 CH | 80.1 CH | 80.1 CH | 79.5 CH | 80.1 CH |
| 17 | 61.6 CH | 62.2 CH | 62.3 CH | 62.2 CH | 61.4 CH |
| 18 | 180.9 C | 182.6 C | 182.8 C | 181.6 C | 182.8 C |
| 19 | 23.9 CH3 | 23.7 CH3 | 23.8 CH3 | 23.9 CH3 | 23.8 CH3 |
| 20 | 81.9 C | 71.3 C | 71.5 C | 71.1 C | 71.7 C |
| 21 | 28.0 CH3 | 26.0 CH3 | 26.0 CH3 | 26.5 CH3 | 26.4 CH3 |
| 22 | 37.7 CH2 | 38.6 CH2 | 42.0 CH2 | 42.9 CH2 | 45.8 CH2 |
| 23 | 26.8 CH2 | 29.4 CH2 | 21.7 CH2 | 22.7 CH2 | 142.8 CH |
| 24 | 86.7 CH | 75.4 CH | 38.1 CH2 | 125.1 CH | 121.9 CH |
| 25 | 70.1 C | 148.4 C | 145.9 C | 131.0 C | 69.9 C |
| 26 | 26.3 CH3 | 110.6 CH2 | 110.3 CH2 | 25.5 CH3 | 29.9 CH3 |
| 27 | 26.7 CH3 | 17.7 CH3 | 22.2 CH3 | 17.4 CH3 | 29.9 CH3 |
| 30 | 17.1 CH3 | 17.0 CH3 | 17.1 CH3 | 17.1 CH3 | 17.0 CH3 |
| 31 | 28.5 CH3 | 28.4 CH3 | 28.5 CH3 | 28.5 CH3 | 28.5 CH3 |
| 32 | 34.3 CH3 | 34.3 CH3 | 34.3 CH3 | 34.3 CH3 | 34.5 CH3 |
| Compound | Hemolytic Activity, ED50, μM/mL * | Cytotoxic Activity, IC50, μM/mL ** | |
|---|---|---|---|
| spleenocytes | Ehrlich carcinoma cells | ||
| 1 | 90.18 ± 1.57 | >100.00 | >100.00 |
| 2 | 33.33 ± 0.48 | 94.18 ± 1.18 | >100.00 |
| 3 | 12.53 ± 0.29 | 19.20 ± 0.03 | 18.95 ± 0.03 |
| 4 | 20.12 ± 0.14 | 37.64 ± 0.00 | 28.37 ± 0.42 |
| 5 | 49.57 ± 0.63 | >100.00 | >100.00 |
| 6 | 58.11 ± 0.69 | >100.00 | >100.00 |
| 7 | 6.97 ± 0.14 | 8.97 ± 0.04 | 18.65 ± 0.00 |
| 8 | 16.20 ± 0.49 | 17.31 ± 0.43 | 37.52 ± 0.00 |
| 9 | 6.52 ± 0.16 | 12.23 ± 0.33 | 35.06 ± 0.18 |
| Typicosid A1 | 3.15 ± 0.12 | 9.92 ± 0.12 | 35.66 ± 0.11 |
| Cucumarioside A2-2 | 0.82 ± 0.02 | 1.21 ± 0.02 | 1.39 ± 0.015 |
| Glycoside | IC50, µM * | Glycoside | IC50, µM * |
|---|---|---|---|
| magnumoside A1 (1) | >100 | magnumoside B2 (6) | >100 |
| magnumoside A2 (2) | >100 | magnumoside C1 (7) | 32.9 |
| magnumoside A3 (3) | 30.3 | magnumoside C2 (8) | 37.1 |
| magnumoside A4 (4) | >100 | magnumoside C4 (9) | 33.9 |
| magnumoside B1 (5) | >100 | – | – |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Kalinin, V.I.; Andrijaschenko, P.V.; Dmitrenok, P.S.; Chingizova, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dautova, T.N. Nine New Triterpene Glycosides, Magnumosides A1–A4, B1, B2, C1, C2 and C4, from the Vietnamese Sea Cucumber Neothyonidium (=Massinium) magnum: Structures and Activities against Tumor Cells Independently and in Synergy with Radioactive Irradiation. Mar. Drugs 2017, 15, 256. https://doi.org/10.3390/md15080256
Silchenko AS, Kalinovsky AI, Avilov SA, Kalinin VI, Andrijaschenko PV, Dmitrenok PS, Chingizova EA, Ermakova SP, Malyarenko OS, Dautova TN. Nine New Triterpene Glycosides, Magnumosides A1–A4, B1, B2, C1, C2 and C4, from the Vietnamese Sea Cucumber Neothyonidium (=Massinium) magnum: Structures and Activities against Tumor Cells Independently and in Synergy with Radioactive Irradiation. Marine Drugs. 2017; 15(8):256. https://doi.org/10.3390/md15080256
Chicago/Turabian StyleSilchenko, Alexandra S., Anatoly I. Kalinovsky, Sergey A. Avilov, Vladimir I. Kalinin, Pelageya V. Andrijaschenko, Pavel S. Dmitrenok, Ekaterina A. Chingizova, Svetlana P. Ermakova, Olesya S. Malyarenko, and Tatyana N. Dautova. 2017. "Nine New Triterpene Glycosides, Magnumosides A1–A4, B1, B2, C1, C2 and C4, from the Vietnamese Sea Cucumber Neothyonidium (=Massinium) magnum: Structures and Activities against Tumor Cells Independently and in Synergy with Radioactive Irradiation" Marine Drugs 15, no. 8: 256. https://doi.org/10.3390/md15080256




