Trichophycin A, a Cytotoxic Linear Polyketide Isolated from a Trichodesmium thiebautii Bloom
Abstract
:1. Introduction
2. Results
2.1. Structure Elucidation of 1
2.2. Cyotoxicity of Trichophycin A
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Biological Material
4.3. Extraction and Isolation of 1
4.4. Cytotoxicity Assays
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kehr, J.C.; Picchi, D.G.; Dittmann, E. Natural product biosynthesis in cyanobacteria: A treasure trove of unique enzymes. Beilstein J. Org. Chem. 2011, 7, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Calteau, A.; Fewer, A.; Latifi, A.; Coursin, T.; Laurent, T.; Jokela, J.; Kerfeld, C.A.; Sivonen, K.; Piel, J.; Gugger, M. Phylum-wide comparative genomics unravel the diversity of secondary metabolism in Cyanobacteria. BMC Genom. 2014, 15, 997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.T. Pharmaceutical agents from filamentous marine cyanobacteria. Drug Discov. Today 2013, 18, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Kale, A.J.; Fenley, A.T.; Byrum, T.; Debonsi, H.M.; Gilson, M.K.; Valeriote, F.A.; Moore, B.S.; Gerwick, W.H. The carmaphycins: New proteasome inhibitors exhibiting an α,β-epoxyketone warhead from a marine cyanobacterium. ChemBioChem 2012, 13, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.; Pereira, A.R.; Debonsi, H.M.; Ligresti, A.; Di Marzo, V.; Gerwick, W.H. Cannabinomimetic lipid from a marine cyanobacterium. J. Nat. Prod. 2011, 74, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Eksioglu, E.A.; Liu, C.; Paul, V.J.; Luesch, H. Grassystatins A-C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation. J. Med. Chem. 2009, 52, 5732–5747. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Corbett, T.H. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 2001, 123, 5418–5423. [Google Scholar] [CrossRef] [PubMed]
- Bertin, M.J.; Demirkiran, O.; Navarro, G.; Moss, N.A.; Lee, J.; Goldgof, G.M.; Vigil, E.; Winzeler, E.A.; Valeriote, F.A.; Gerwick, W.H. Kalkipyrone B, a marine cyanobacterial γ-pyrone possessing cytotoxic and anti-fungal activities. Phytochemistry 2016, 122, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Reyes, L.A.; Sneed, J.; Paul, V.J.; Luesch, H. Amantelides A and B, polyhydroxylated macrolides with differential broad-spectrum cytotoxicity from a Guamanian marine cyanobacterium. J. Nat. Prod. 2015, 78, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Cummings, S.; Lee, J.; Moss, N.; Glukhov, E.; Valeriote, F.A.; Gerwick, L.; Gerwick, W.H. Isolation of polycavernoside D from a marine cyanobacterium. Environ. Sci. Technol. Lett. 2015, 2, 166–170. [Google Scholar] [CrossRef]
- Nunnery, J.K.; Engene, N.; Byrum, T.; Cao, Z.; Jabba, S.V.; Pereira, A.R.; Matainaho, T.; Murray, T.F.; Gerwick, W.H. Biosynthetically intriguing chlorinated lipophilic metabolites from geographically distant tropical marine cyanobacteria. J. Org. Chem. 2012, 77, 4198–4208. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Grosso, M.F.; Villa, F.A.; Engene, N.; McPhail, K.L.; Tidgewell, K.; Pineda, L.M.; Gerwick, L.; Spadafora, C.; Kyle, D.E.; et al. Coibacins A-D, antileishmanial marine cyanobacterial polyketides with intriguing biosynthetic origins. Org. Lett. 2012, 14, 5543–5554. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.T.; Boulanger, A.; Vulpanovici, A.; Roberts, M.A.; Gerwick, W.H. Structure and absolute stereochemistry of phormidolide, a new toxic metabolite from the marine cyanobacterium Phormidium sp. J. Org. Chem. 2002, 67, 7927–7936. [Google Scholar] [CrossRef] [PubMed]
- Karl, D.; Michaels, A.; Bergman, B.; Capone, D.; Carpenter, E.; Letelier, R.; Lipschultz, F.; Paerl, H.; Sigman, D.; Stal, L. Dinitrogen fixation in the world’s oceans. Biogeochemistry 2002, 57/58, 47–98. [Google Scholar] [CrossRef]
- Bergman, B.; Sandh, G.; Lin, S.; Larsson, J.; Carpenter, E.J. Trichodesmium—A widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiol. Rev. 2013, 37, 286–302. [Google Scholar] [CrossRef] [PubMed]
- Westberry, T.K.; Siegel, D.A. Spatial and temporal distribution of Trichodesmium blooms in the world’s oceans. Glob. Biogeochem. Cycles 2006, 20, GB4016. [Google Scholar] [CrossRef]
- Mulholland, M.R.; Bernhardt, P.W.; Heil, C.A.; Bronk, D.A.; O’Neil, J.M. Nitrogen fixation and release of fixed nitrogen by Trichodesmium spp. in the Gulf of Mexico. Limnol. Oceanogr. 2006, 51, 1762–1776. [Google Scholar] [CrossRef]
- Walsh, J.J.; Weisberg, R.H.; Lenes, J.M.; Chen, F.R.; Dieterle, D.A.; Zheng, L.; Carder, K.L.; Vargo, G.A.; Havens, J.A.; Peebles, E.; et al. Isotopic evidence for dead fish maintenance of Florida red tides, with implications for coastal fisheries over both source regions of the West Florida shelf and within downstream waters of the South Atlantic Bight. Prog. Oceanogr. 2009, 80, 51–73. [Google Scholar] [CrossRef]
- Hawser, S.P.; O’Neil, J.M.; Roman, M.R.; Codd, G.A. Toxicity of blooms of the cyanobacterium Trichodesmium to zooplankton. J. Appl. Phycol. 1992, 4, 79–86. [Google Scholar] [CrossRef]
- Guo, C.; Tester, P.A. Toxic effect of the bloom-forming Trichodesmium sp. (Cyanophyta) to the copepod Acartia tonsa. Nat. Toxins 1994, 2, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ohki, K.; Fujita, Y. Laboratory culture of the pelagic blue-green alga Trichodesmium thiebautii: Conditions for unialgal culture. Mar. Ecol. Prog. Ser. 1982, 7, 185–190. [Google Scholar] [CrossRef]
- Sudek, S.; Haygood, M.G.; Youssef, D.T.A.; Schmidt, E.W. Structure of trichamide, a cyclic peptide from the bloom-forming cyanobacterium Trichodesmium erythraeum, predicted from the genome sequence. Appl. Environ. Microbiol. 2006, 72, 4382–4387. [Google Scholar] [CrossRef] [PubMed]
- Malloy, K.L.; Suyama, T.L.; Engene, N.; Debonsi, H.; Cao, Z.; Matainaho, T.; Spadafora, C.; Murray, T.F.; Gerwick, W.H. Credneramides A and B: Neuromodulatory phenethylamine and isopentylamine derivatives of a vinyl chloride-containing fatty acid from cf. Trichodesmium sp. nov. J. Nat. Prod. 2012, 75, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Schock, T.B.; Huncik, K.; Beauchesne, K.R.; Villareal, T.A.; Moeller, P.D.R. Identification of trichotoxin, a novel chlorinated compound associated with the bloom forming cyanobacterium, Trichodesmium thiebautii. Environ. Sci. Technol. 2011, 45, 7503–7509. [Google Scholar] [CrossRef] [PubMed]
- Bertin, M.J.; Zimba, P.V.; He, H.; Moeller, P.D.R. Structure revision of trichotoxin, a chlorinated polyketide isolated from a Trichodesmium thiebautii bloom. Tetrahedron Lett. 2016, in press. [Google Scholar] [CrossRef]
- Edwards, D.J.; Marquez, B.L.; Nogle, L.M.; McPhail, K.; Goeger, D.E.; Roberts, M.A.; Gerwick, W.H. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem. Biol. 2004, 11, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Matthews, J.H.; Paul, V.J.; Luesch, H. Pitiamides A and B, multifunctional fatty acid amides from marine cyanobacteria. Planta Med. 2016, 82, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Keatinge-Clay, A.T.; Stroud, R.M. The structure of a ketoreductase determines the organization of the β-carbon processing enzymes of modular polyketide synthases. Structure 2006, 14, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, S.A.; Whicher, J.R.; Papireddy, K.; Florova, G.; Smith, J.L.; Reynolds, K.A. Structural and stereochemical analysis of a modular polyketide synthase ketoreductase domain required for the generation of cis-alkene. Chem. Biol. 2013, 20, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Keatinge-Clay, A.T. The status of type I polyketide synthase ketoreductases. Med. Chem. Commun. 2013, 4, 34–40. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Cyanoprokarota 19 Part 2: Oscillatoriales; Elsevier: München, Germany, 2005; pp. 1–759. [Google Scholar]
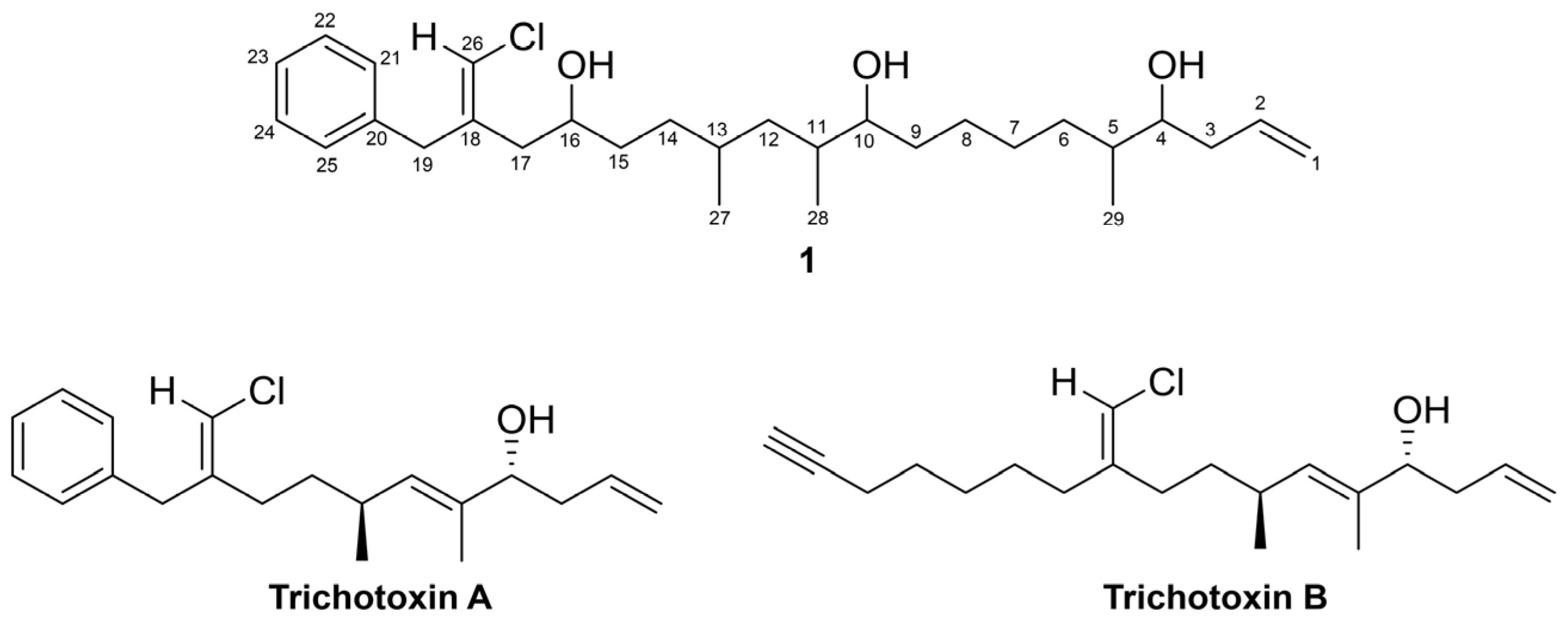
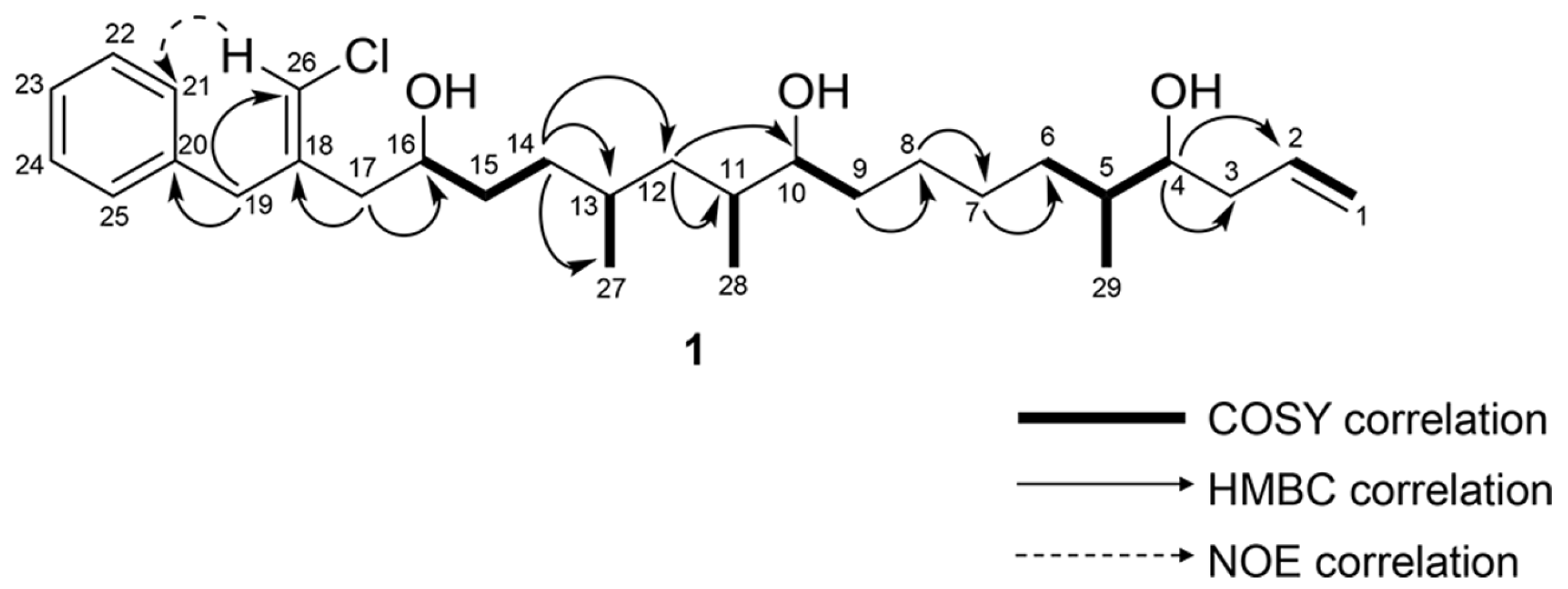

| Pos | δC, Type | δH (J in Hz) | HMBC | COSY | NOESY |
|---|---|---|---|---|---|
| 1a | 117.9, CH2 | 5.15, m | 2, 3 | 2 | |
| 1b | 5.12, m | 2, 3 | 2 | ||
| 2 | 135.6, CH | 5.83, m | 3, 4 | 1a, 1b, 3a, 3b | |
| 3a | 39.1, CH2 | 2.26, m | 1, 2, 4, 5 | 2, 3b, 4 | 29 |
| 3b | 2.16, m | 1, 2, 4, 5 | 2, 3a, 4 | 6b, 29 | |
| 4 | 73.9, CH | 3.55, dt (8.9, 4.0) | 2, 3, 5, 6, 29 | 3a, 3b, 5 | 6b, 7b, 29 |
| 5 | 37.8, CH | 1.53, ovlp a | 4, 29 | 4, 6b, 29 | 3a, 3b |
| 6a | 33.0, CH2 | 1.48, m | 4, 5, 7, 8, 29 | 6b, 7a, 7b | 3b |
| 6b | 1.19, m | 4, 5, 7, 8, 29 | 5, 6a | 3b, 4 | |
| 7a | 27.4, CH2 | 1.38, ovlp | 5, 6, 8 | ||
| 7b | 1.32, ovlp | 6, 8 | 4 | ||
| 8a | 26.7, CH2 | 1.44, ovlp | 7, 9 | ||
| 8b | 1.32, ovlp | 6, 7, 10 | |||
| 9 | 34.6, CH2 | 1.44, ovlp | 10 | 10 | |
| 10 | 74.5, CH | 3.50, m | 8, 9, 11, 12, 28 | 9, 11 | 12b, 13, 28 |
| 11 | 35.2, CH | 1.59, m | 9, 10, 12, 13, 28 | 10, 12a, 12b, 28 | |
| 12a | 40.8, CH2 | 1.39, ovlp | 10, 11, 13, 14 27, 28 | 12b | |
| 12b | 1.00, m | 10, 11, 13, 14, 27, 28 | 11, 12a | 10 | |
| 13 | 29.8, CH | 1.52, ovlp | 12, 14, 27 | 12b, 14b, 27 | 10, 16 |
| 14a | 32.5, CH2 | 1.35, ovlp | 12, 13, 15, 16, 27 | 14b, 15b | 16, 17b |
| 14b | 1.22, m | 12, 13, 15, 16, 27 | 14a, 15b | 16, 17b | |
| 15a | 34.8, CH2 | 1.52, ovlp | 13, 14, 16, 17 | 14a, 14b, 16 | 17a, 17b |
| 15b | 1.44, ovlp | 13, 14, 16, 17 | 14b, 16 | 17a, 17b | |
| 16 | 70.7, CH | 3.79, m | 14, 15, 17, 18 | 15a, 15b, 17a, 17b | 4a, 13, 14b, 27 |
| 17a | 38.2, CH2 | 2.38, dd (13.7, 8.8) | 15, 16, 18, 19, 26 | 16, 17b | 15a, 15b, 19 |
| 17b | 2.28, dd (13.7, 4.2) | 15, 16, 18, 19, 26 | 16, 17a | 15a, 15b, 19 | |
| 18 | 139.5, qC | ||||
| 19 | 41.9, CH2 | 3.46, d (5.5) | 17, 18, 20, 21, 25, 26 | 17a, 17b, 26 | |
| 20 | 138.0, qC | ||||
| 21 | 129.0, CH | 7.17, d (7.6) | 19, 22, 23 | 22 | 19, 26 |
| 22 | 128.6, CH | 7.30, t (7.6) | 20 | 21 | |
| 23 | 126.7, CH | 7.23, t (7.6) | 22, 24 | 22, 24 | |
| 24 | 128.6, CH | 7.30, t (7.6) | 20 | 25 | |
| 25 | 129.0, CH | 7.17, d (7.6) | 19, 23, 24 | 24 | 19 |
| 26 | 116.2, CH | 5.99, s | 16, 17, 18, 19 | 19, 21 | |
| 27 | 20.4, CH3 | 0.87, d (6.6) | 12, 13, 14 | 13 | 10, 11, 14b, 16 |
| 28 | 13.9, CH3 | 0.84, d (6.8) | 10, 11, 12 | 11 | 9, 10 |
| 29 | 13.9, CH3 | 0.90, d (6.8) | 4, 5, 6 | 5 | 3a, 3b, 4, 6b |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertin, M.J.; Wahome, P.G.; Zimba, P.V.; He, H.; Moeller, P.D.R. Trichophycin A, a Cytotoxic Linear Polyketide Isolated from a Trichodesmium thiebautii Bloom. Mar. Drugs 2017, 15, 10. https://doi.org/10.3390/md15010010
Bertin MJ, Wahome PG, Zimba PV, He H, Moeller PDR. Trichophycin A, a Cytotoxic Linear Polyketide Isolated from a Trichodesmium thiebautii Bloom. Marine Drugs. 2017; 15(1):10. https://doi.org/10.3390/md15010010
Chicago/Turabian StyleBertin, Matthew J., Paul G. Wahome, Paul V. Zimba, Haiyin He, and Peter D. R. Moeller. 2017. "Trichophycin A, a Cytotoxic Linear Polyketide Isolated from a Trichodesmium thiebautii Bloom" Marine Drugs 15, no. 1: 10. https://doi.org/10.3390/md15010010
APA StyleBertin, M. J., Wahome, P. G., Zimba, P. V., He, H., & Moeller, P. D. R. (2017). Trichophycin A, a Cytotoxic Linear Polyketide Isolated from a Trichodesmium thiebautii Bloom. Marine Drugs, 15(1), 10. https://doi.org/10.3390/md15010010





