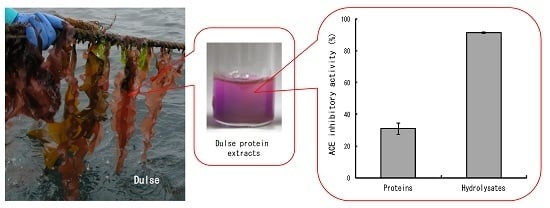
Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata
Abstract
:1. Introduction
2. Results and Discussion
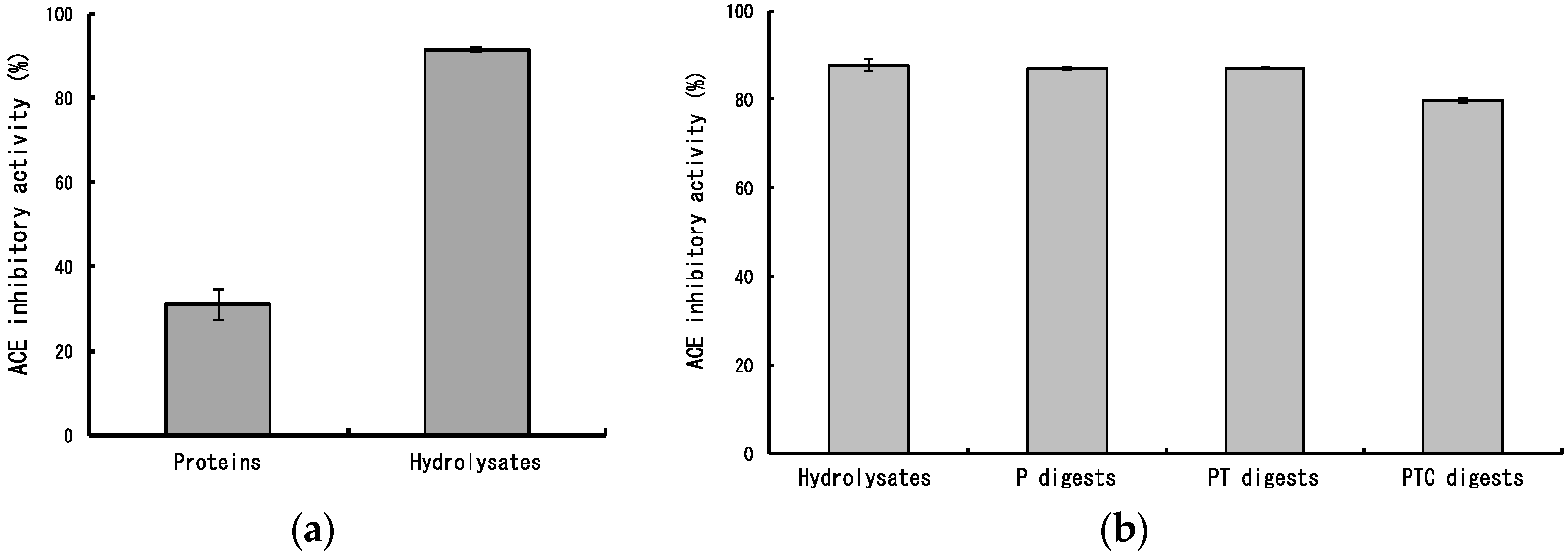
2.1. ACE Inhibitory Activity of Dulse Protein Hydrolysates
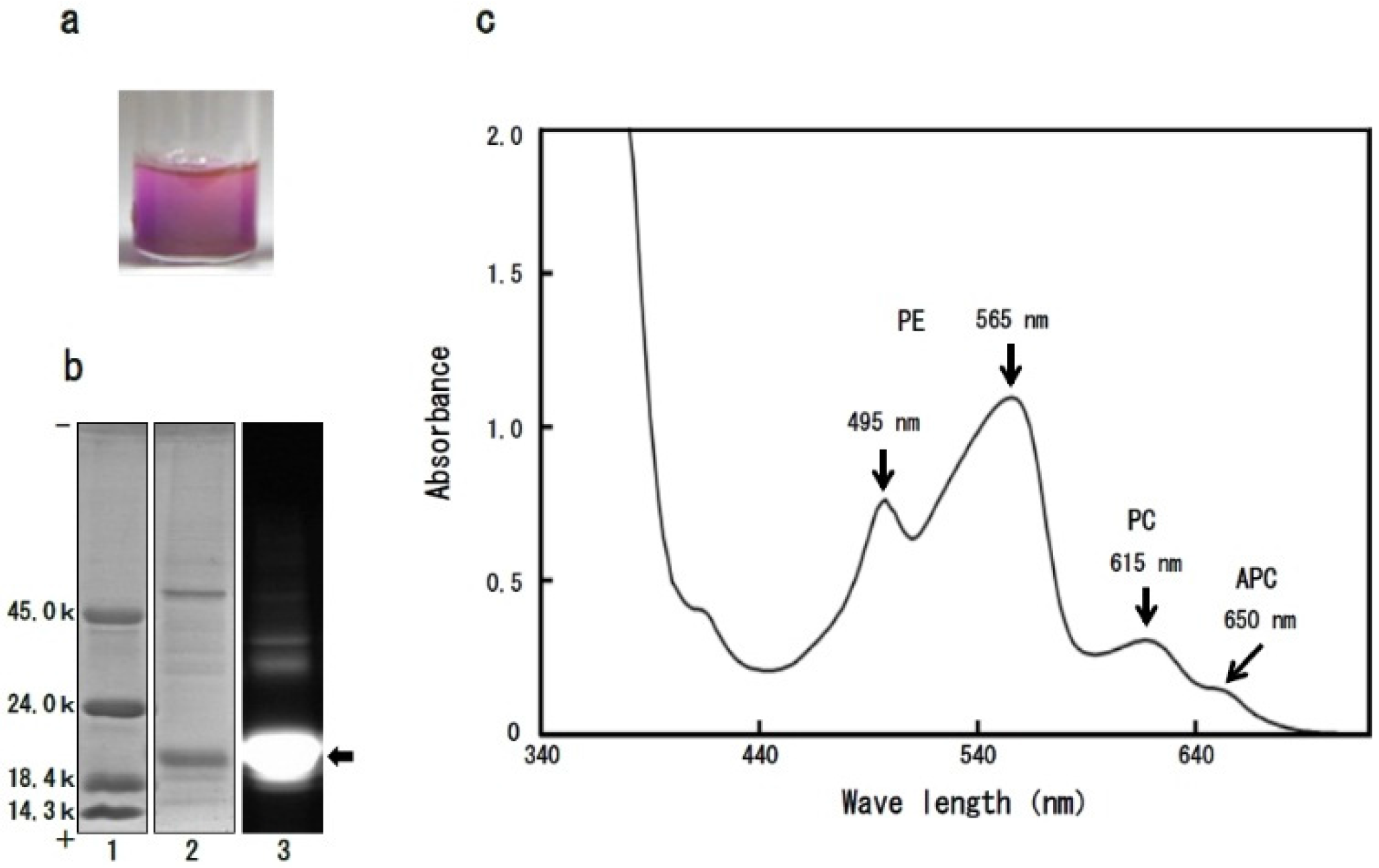
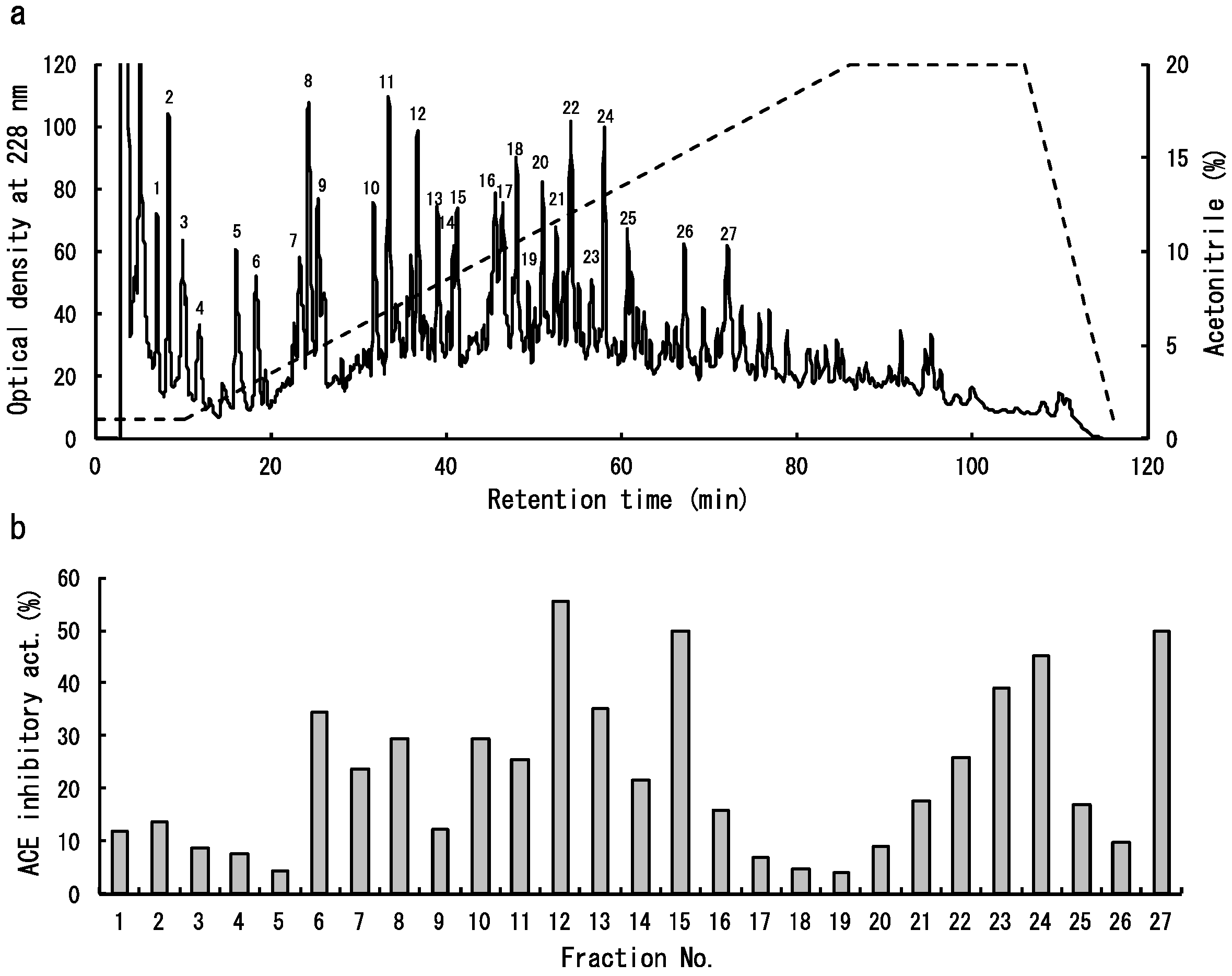
2.2. Isolation of Dulse ACE Inhibitory Peptides
| Fraction No. | Amino Acid Sequence |
|---|---|
| 6 | Not determined |
| 7 | Not determined |
| 8 | YRD |
| 10 | AGGEY |
| 11 | Not determined |
| 12 | VYRT |
| 13 | VDHY |
| 15 | IKGHY |
| LKNPG | |
| 23 | Not determined |
| 24 | LDY |
| LRY | |
| 27 | FEQDWAS |
| Amino Acid Sequence | IC50 Value (μmol) |
|---|---|
| Dulse (in this study) | |
| VYRT | 0.14 |
| LDY | 6.1 |
| LRY | 0.044 |
| FEQDWAS | >2.8 |
| Chum salmon | |
| IW | 0.024 * |
| Sesame | |
| LVY | 0.045 ** |
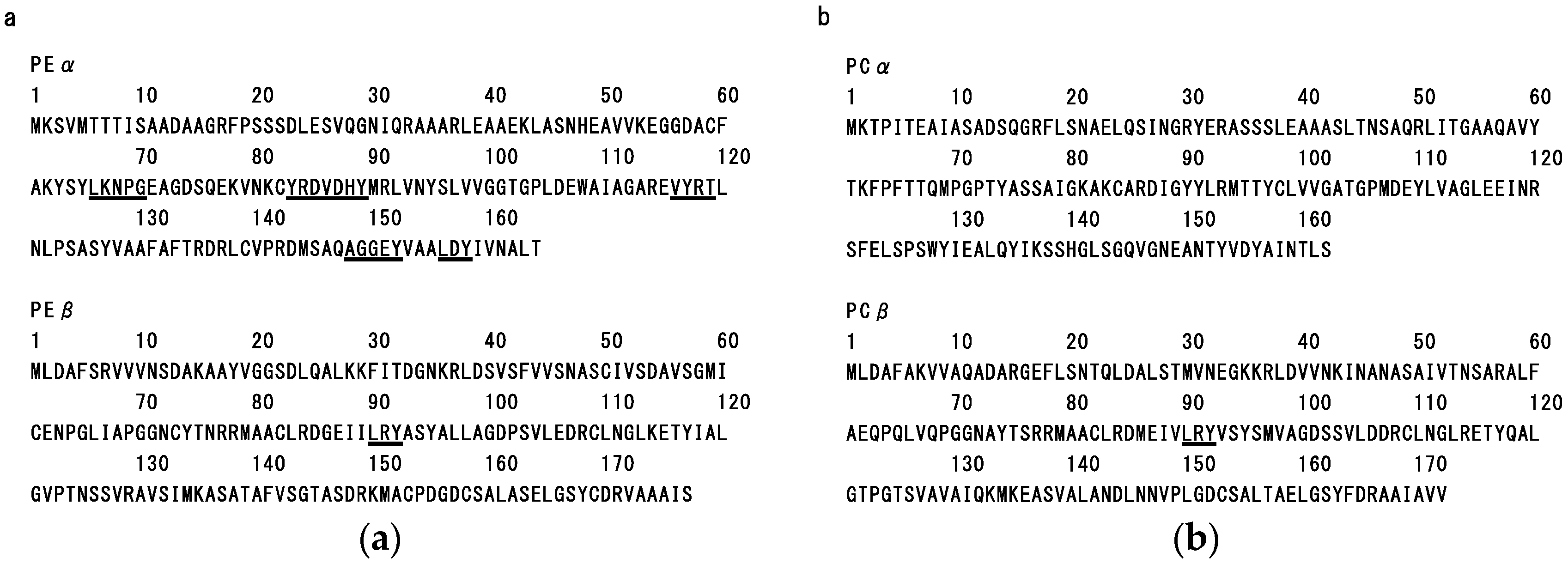
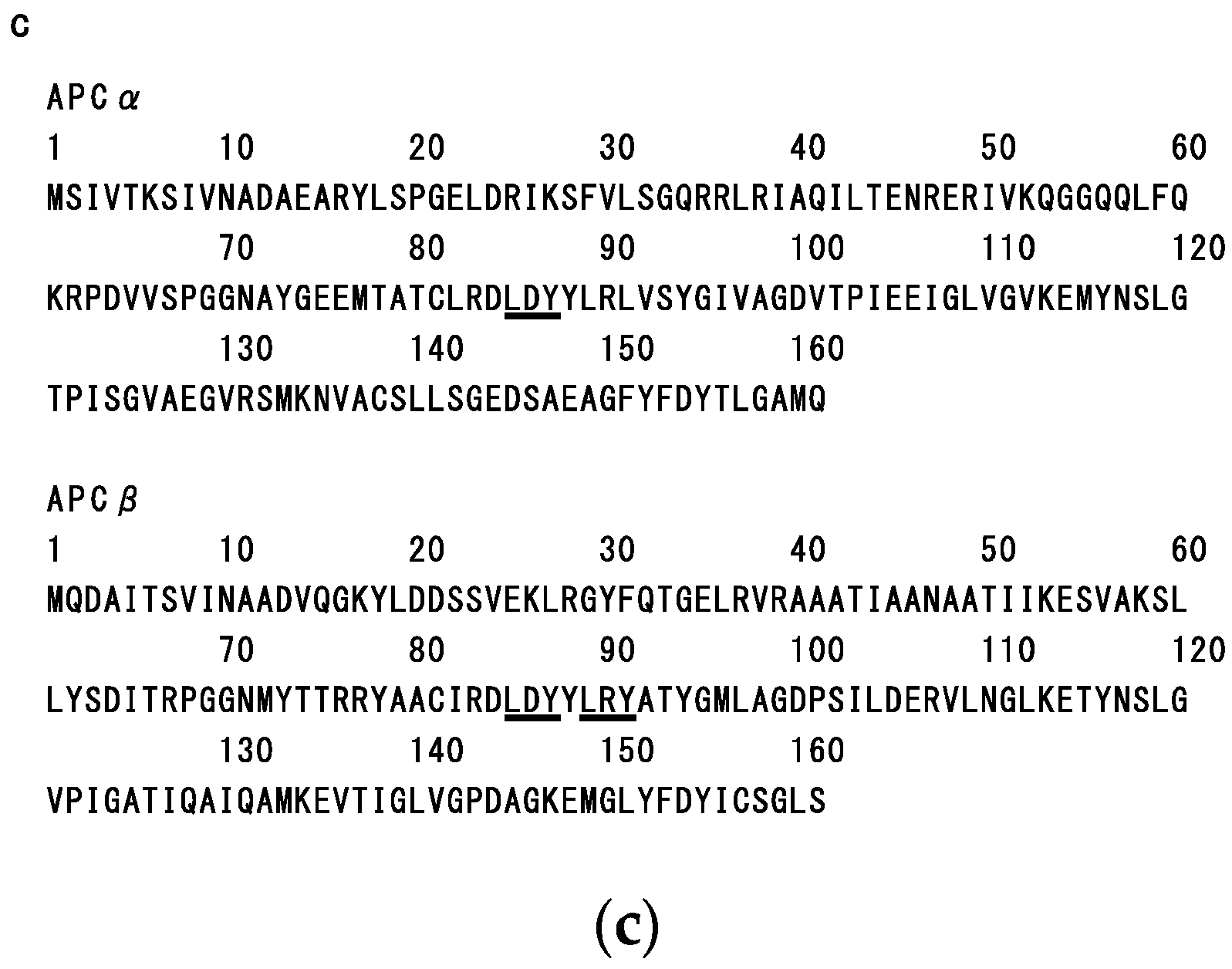
2.3. Structure-Function Relationship of Dulse Phycobiliproteins
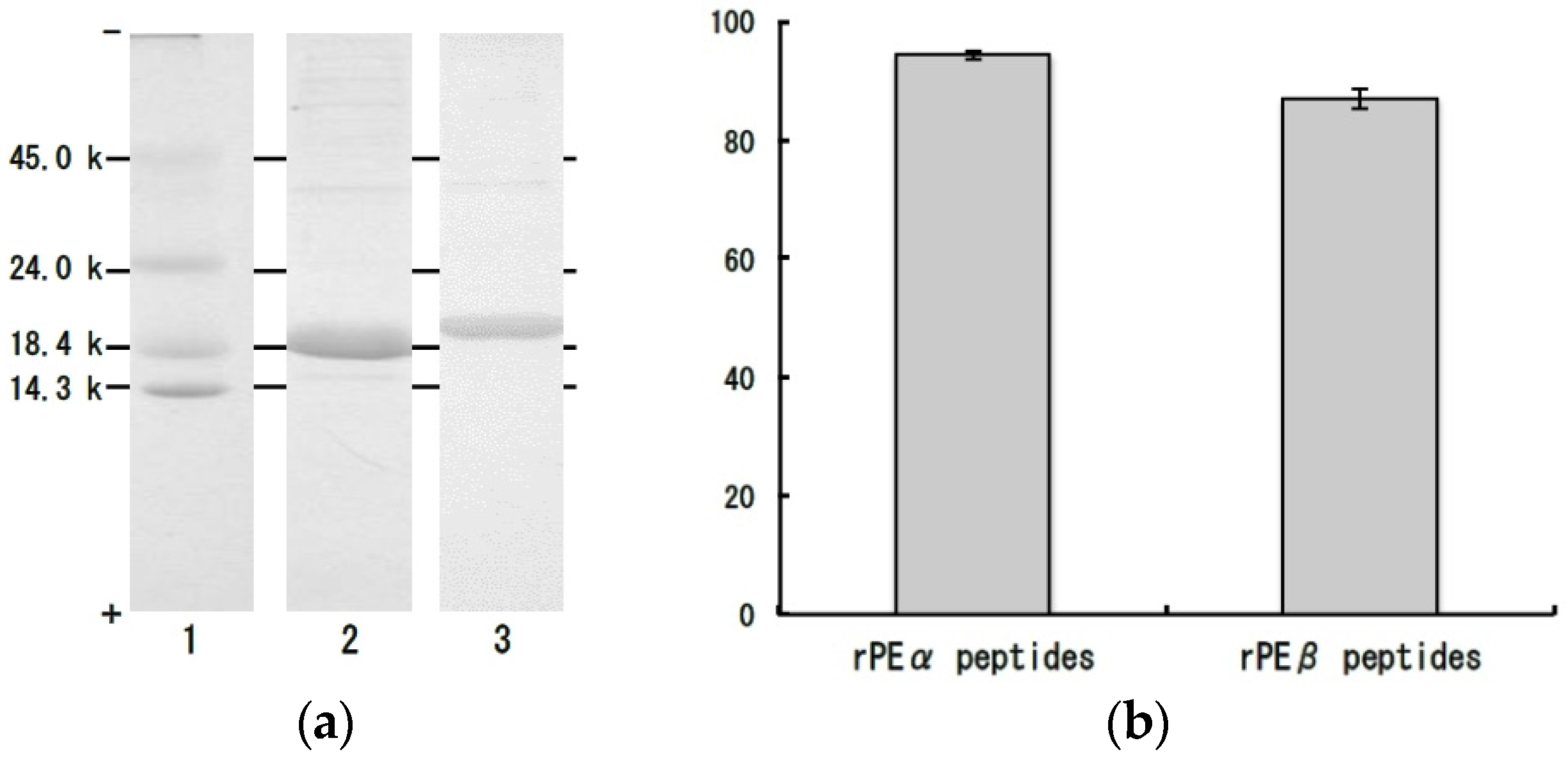
3. Experimental Section
3.1. Materials
3.2. Preparation of Protein Hydrolysates from Dulse
3.3. Isolation of Peptides from Dulse Protein Hydrolysates
3.4. ACE Inhibitory Assay
3.5. Analysis of Visible Ray Absorption Spectrum of Dulse Proteins
3.6. Polyacrylamide Gel Electrophoresis of Dulse Proteins
3.7. Analysis of Amino Acid Sequences of Dulse ACE Inhibitory Peptides
3.8. Bacterial Expression of Recombinant α- and β-Subunits of Dulse Phycoerythrin
| Primer | Sequence (5′→3′) |
|---|---|
| RDPEαF | GGAGATTACCATGGAATCAG |
| RDPEαR | AAGGGATCATTAGGTTAAAG |
| RDPEβF | TAAGGAGAGTTBCATGGTTG |
| RDPEβR | AACAGGATCCATTAGCTAATTGCAGC |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheung, H.-S.; Wang, F.-L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme: Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980, 25, 401–407. [Google Scholar]
- Balti, R.; Bougatef, A.; Sila, A.; Guillochon, D.; Dhulster, P.; Nedjar-Arroume, N. Nine novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, P.J.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Production and identification of angiotensin I-converting enzyme (ACE) inhibitory peptides from Mediterranean fish discards. J. Funct. Foods 2015, 18, 95–105. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; González, P.; Esteban-Fernández, D.; Carrera, M.; Piñeiro, C. Identification of the major ACE-inhibitory peptides produced by enzymatic hydrolysis of a protein concentrate from cuttlefish wastewater. Mar. Drugs 2014, 12, 1390–1405. [Google Scholar] [CrossRef] [PubMed]
- Ghassem, M.; Babji, A.S.; Said, M.; Mahmoodani, F.; Aeihara, K. Angiotensin I-converting enzyme inhibitory peptides from snakehead fish sarcoplasmic protein hydrolysate. J. Food Biochem. 2014, 38, 140–149. [Google Scholar] [CrossRef]
- Kuba, M.; Tana, C.; Tawata, S.; Yasuda, M. Production of angiotensin I-converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase. Process Biochem. 2005, 40, 2191–2196. [Google Scholar] [CrossRef]
- Tsai, J.S.; Chen, T.J.; Pan, B.S.; Gong, S.D.; Chung, M.Y. Antihypertensive effect of bioactive peptides produced by protease facilitated lactic acid fermentation of milk. Food Chem. 2008, 106, 552–558. [Google Scholar] [CrossRef]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Maekawa, K.; Chen, J.-R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Lee, K.-W.; Jeon, Y.-J. Screening of extracts from red algae in Jeju for potentials marine angiotensin-I converting enzyme (ACE) inhibitory activity. Algae 2006, 21, 343–348. [Google Scholar] [CrossRef]
- He, H.-L.; Chen, X.-L.; Wu, H.; Sun, C.-Y.; Zhang, Y.-Z.; Zhou, B.-C. High throughput and rapid screening of marine protein hydrolysates enriched in peptides with angiotensin-I-converting enzyme inhibitory activity activity by capillary electrophoresis. Bioresour. Thechnol. 2007, 98, 3499–3505. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Mora-Soler, L.; Gallagher, E.; O’Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and characterization of bioactive pro-peptides with in vitro renin inhibitory activities from the macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Purification and identification of dipeptidyl peptidase (DPP) IV inhibitory peptides from the macroalga Palmaria palmata. Food Chem. 2015, 172, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Apt, K.E.; Collier, J.L.; Grossman, A.R. Evolution of the phycobiliproteins. J. Mol. Biol. 1995, 248, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Gong, X.; Zhao, M.; Fu, X.; Wang, L. Isolation, purification and characteristics of R-phycoerythrin from a marine macroalga Heterosiphonia japonica. Protein Expr. Purif. 2009, 64, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Saiga, A.; Iwai, K.; Hayakawa, T.; Takahata, Y.; Kitamura, S.; Nishimura, T.; Morimatsu, F. Angiotensin I-converting enzyme-inhibitory peptides obtained from chicken collagen hydrolysate. J. Agric. Food Chem. 2008, 56, 9586–9591. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Hosokawa, M.; Miyashita, K.; Takahashi, K. Isolation of peptides with angiotensin I-converting enzyme inhibitory effect derived from hydrolysate of upstream chum salmon muscle. J. Food Sci. 2003, 68, 1611–1614. [Google Scholar] [CrossRef]
- Nakano, D.; Ogura, K.; Miyakoshi, M.; Ishii, F.; Kawanishi, H.; Kurumazuka, D.; Kwak, C.-J.; Ikemura, K.; Takaoka, M.; Moriguchi, S.; et al. Antihypertensive effect of angiotensin I-converting enzyme inhibitory peptides from a sesame protein hydrolysate in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 70, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Cushman, D.W. Inhibition of homogeneous angiotensin-converting enzyme of rabbit lung by synthetic venom peptides of Bothrops jararaca. Biochim. Biophys. Acta 1973, 293, 451–463. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kishimura, H.; Ojima, T.; Hayashi, K.; Nishita, K. Bacterial expression and characterization of the starfish phospholipase A2. Comp. Biochem. Physiol. 2001, 128, 565–573. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. https://doi.org/10.3390/md14020032
Furuta T, Miyabe Y, Yasui H, Kinoshita Y, Kishimura H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Marine Drugs. 2016; 14(2):32. https://doi.org/10.3390/md14020032
Chicago/Turabian StyleFuruta, Tomoe, Yoshikatsu Miyabe, Hajime Yasui, Yasunori Kinoshita, and Hideki Kishimura. 2016. "Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata" Marine Drugs 14, no. 2: 32. https://doi.org/10.3390/md14020032
APA StyleFuruta, T., Miyabe, Y., Yasui, H., Kinoshita, Y., & Kishimura, H. (2016). Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Marine Drugs, 14(2), 32. https://doi.org/10.3390/md14020032