Biological Activities and Chemical Composition of Methanolic Extracts of Selected Autochthonous Microalgae Strains from the Red Sea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity
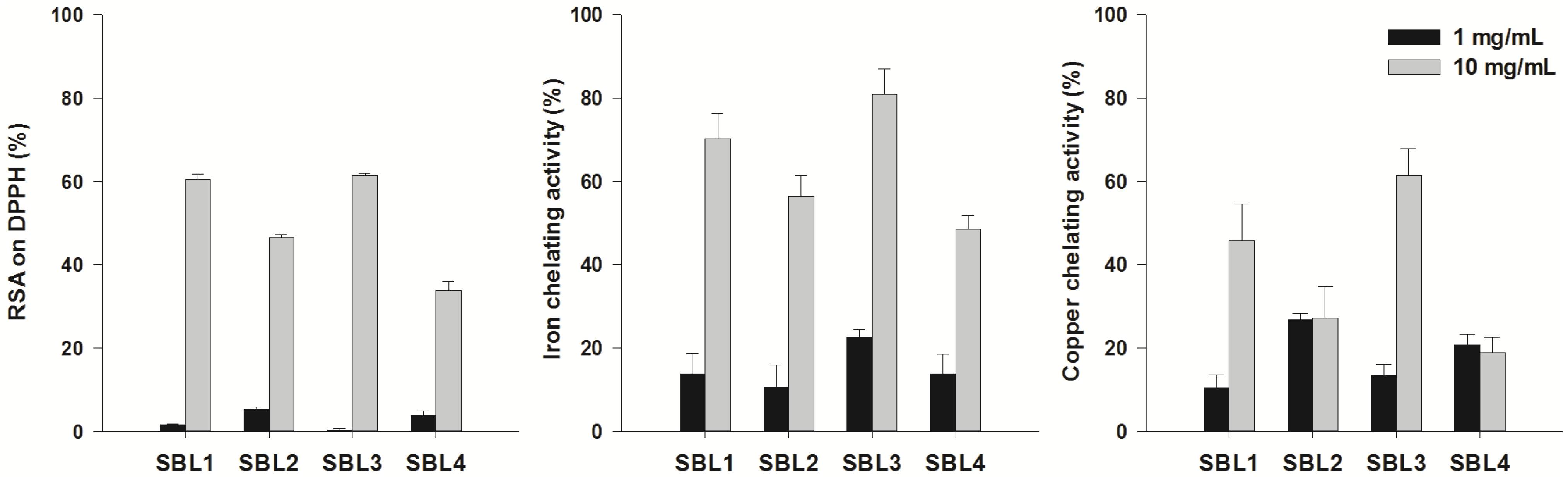
2.2. Acetylcholinesterase, Butyrylcholinesterase and Tyrosinase Inhibitory Activity
| AChE | BuChE | TYRO | ||||
|---|---|---|---|---|---|---|
| Species/Standard | 1 mg/mL | 10 mg/mL | 1 mg/mL | 10 mg/mL | 1 mg/mL | 10 mg/mL |
| SBL1 | na | 17.1 ± 5.7 | 52.0 ± 8.4 | 58.0 ± 7.4 | 22.7 ± 4.6 | 44.8 ± 5.1 |
| SBL2 | na | 21.2 ± 8.1 | 66.1 ± 3.4 | 69.3 ± 2.5 | 32.6 ± 7.3 | 39.5 ± 5.4 |
| SBL3 | na | na | 55.2 ± 6.5 | 60.4 ± 5.2 | 15.0 ± 5.2 | 40.1 ± 3.5 |
| SBL4 | na | na | 59.0 ± 8.2 | 41.2 ± 12.0 | 10.6 ± 4.1 | na |
| Galantamine * | 93.2 ± 0.5 | nt | 80.3 ± 0.7 | nt | - | - |
| Arbutin * | - | - | - | - | 78.3 ± 0.1 | nt |
2.3. In Vitro Cytotoxic Activity

2.4. In Vitro Antileishmanial Activity
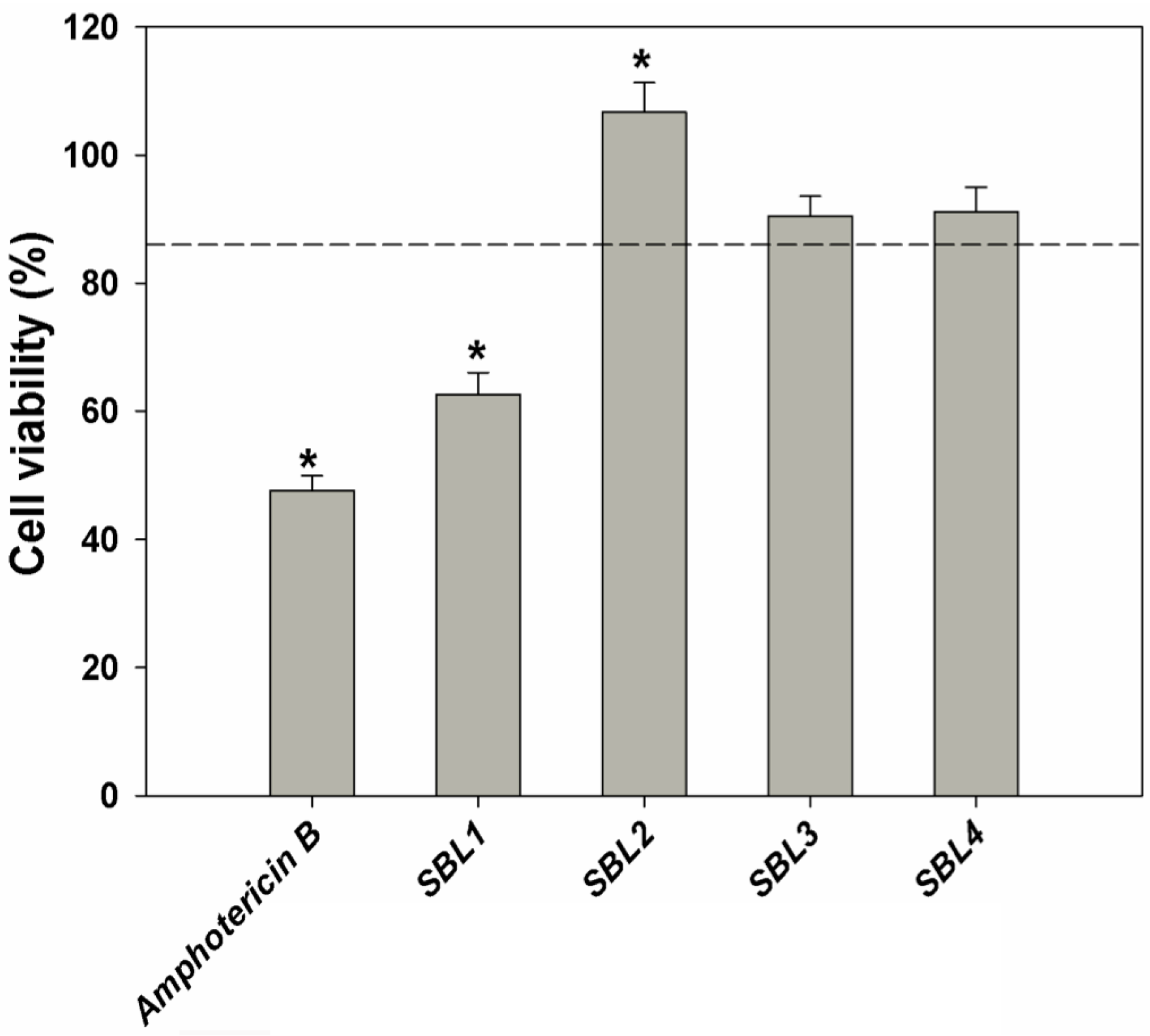
2.5. Extraction Yield and Phytochemical Analysis
| Compound | SBL1 | SBL2 | SBL3 | SBL4 |
|---|---|---|---|---|
| Gallic acid | 0.06 | 0.11 | nd | nd |
| Coumaric acid | 0.06 | 0.35 | 0.07 | 0.07 |
| Salicylic acid | nd | 0.64 | nd | 0.14 |
| Total phenolics | 0.12 | 1.1 | 0.07 | 0.21 |
| Neoxanthin | 0.02 | 1.45 | 0.11 | 0.06 |
| Violaxanthin | 0.16 | 0.44 | 0.05 | 0.15 |
| Lutein | 1.29 | 0.89 | 0.60 | 0.19 |
| Zeaxanthin | 0.51 | 0.54 | 0.48 | 0.10 |
| Canthaxanthin | nd | 1.15 | nd | nd |
| β-carotene | 1.08 | 0.52 | 0.61 | 1.19 |
| Total carotenoids | 3.07 | 3.55 | 1.74 | 1.62 |
3. Experimental Section
3.1. Chemicals
3.2. Microalgae Culture
3.3. Extraction
3.4. Antioxidant Activity
3.4.1. RSA on DPPH Radical
3.4.2. Metal Chelating Activity on Iron and Copper Ions
3.5. AChE and BuChE Inhibitory Activity
3.6. TYRO Inhibitory Activity
3.7. In Vitro Cytotoxic Activity
3.8. In Vitro Antileishmanial Activity
3.9. TPC
3.10. HPLC Analysis
3.10.1. Analysis of Phenolic Compounds
3.10.2. Analysis of Pigment Composition
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Science: Ames, IA, USA, 2004. [Google Scholar]
- Coesel, S.N.; Baumgartner, A.C.; Teles, L.M.; Ramos, A.R.; Henriques, N.M.; Cancela, L.; Varela, J. Nutrient limitation is the main regulatory factor for carotenoid accumulation and for Psy and Pds steady state transcript levels in Dunaliella salina (Chlorophyta) exposed to high light and salt stress. Mar. Biotechnol. 2008, 10, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process. Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Tran, K.-Q.; Ragnar, H.; Giselrød, H.R. Towards sustainable production of biofuels from microalgae. Int. J. Mol. Sci. 2008, 9, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Stephens, E.; Ross, I.L.; King, Z.; Mussgnug, J.H.; Kruse, O.; Posten, C.; Borowitzka, M.A.; Hankamer, B. An economic and technical evaluation of microalgal biofuels. Nat. Biotechnol. 2010, 28, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, R.H.; Barbosa, M.J. An outlook on microalgal biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Barreira, L.; Custódio, L.; Alrokayan, S.; Mouffouk, F.; Varela, J.; Abu-Salah, K.M.; Ben-Hamadou, R. Isolation and fatty acid profile of selected microalgae strains from the Red Sea for biofuel production. Energies 2013, 6, 2773–2783. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Abe, K.; Hattor, H.; Hiran, M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem. 2005, 100, 656–661. [Google Scholar] [CrossRef]
- Konishi, T. Brain oxidative stress as basic target of antioxidant traditional oriental medicines. Neurochem. Res. 2009, 34, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Pan, J.L.; Chen, C.Y.; Chiu, C.C.; Yang, M.H.; Chang, H.W.; Chang, J.S. Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010, 45, 1865–1872. [Google Scholar] [CrossRef]
- Weinreb, O.; Mandel, S.; Bar-Am, O.; Amit, T. Iron-chelating backbone coupled with monoamine oxidase inhibitory moiety as novel pluripotential therapeutic agents for Alzheimer’s disease: A tribute to Moussa Youdim. J. Neural Transm. 2011, 118, 479–492. [Google Scholar] [CrossRef]
- Uttara, B.; Ajay, S.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Vizetto, C.; Pereira, H.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2012, 131, 134–140. [Google Scholar] [CrossRef]
- Gaeta, A.; Hider, R.C. The crucial role of metal ions in neurodegeneration: The basis for a promising therapeutic strategy. Br. J. Pharmacol. 2005, 146, 1041–1059. [Google Scholar] [CrossRef] [PubMed]
- Poggiali, E.; Cassinerio, E.; Zanaboni, L.; Cappellini, M.D. An update on iron chelation therapy. Blood Transfus. 2012, 10, 411–422. [Google Scholar] [PubMed]
- Robert, A.; Liu, Y.; Nguyen, M.; Meunier, B. Regulation of copper and iron homeostasis by metal chelators: A possible chemotherapy for Alzheimer’s disease. Acc. Chem. Res. 2015, 48, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.; Medeiros, K.; Diniz, M.; Batista, L.; Athayde-Filho, P.; Silva, M.; da Cunha, E. Natural products inhibitors of the enzyme acetylcholinesterase. Braz. J. Pharmacogn. 2006, 16, 258–285. [Google Scholar] [CrossRef]
- Vinutha, B.; Prashanth, D.; Salma, K.; Sreeja, S.L.; Pratiti, D.; Padmaja, R.; Radhika, S.; Amit, A.; Venkateshwarlu, K.; Deepak, M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2007, 109, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Soares, F.; Pereira, H.; Rodrigues, M.J.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Botryococcus braunii and Nannochloropsis oculata extracts inhibit cholinesterases and protect human dopaminergic SH-SY5Y cells from H2O2-induced cytotoxicity. J. Appl. Phycol. 2015, 25, 839–848. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.-S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Graybiel, A.; Ragsdale, C.W., Jr. Pseudocholinesterase staining in the primary visual pathway of the macaque monkey. Nature 1982, 299, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Bartorelli, L.; Giraldi, C.; Saccardo, M.; Cammarata, S.; Bottini, G.; Fasanaro, A.M.; Trequattrini, A. Effects of switching from an AChE inhibitor to a dual AChE-BuChE inhibitor in patients with Alzheimer’s disease. Curr. Med. Res. Opin. 2005, 21, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E.; Spiegel, R.; Enz, A.; Veroff, A.; Cutler, N. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: Correlation with cognitive benefit. J. Neural Trans. 2002, 109, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.-Y.; Weng, T.-T.; Lin, G.-Z.; Lu, R.-J.; Jian, S.-Y.; Lin, G. Molecular docking of different inhibitors and activators to butyrylcholinesterase. J. Biomol. Struct. Dyn. 2015, 33, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.H. Molecular design of tyrosinase inhibitors: A critical review of promising novel inhibitors from synthetic origins. Pure Appl. Chem. 2007, 79, 2277–2295. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of high added-value compounds—A brief review of recent work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Rauter, A.P.; Alberício, F.; Varela, J. Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible application in the pharmaceutical and functional food industries. J. Appl. Phycol. 2014, 26, 151–161. [Google Scholar]
- Campino, L.; Pratlong, F.; Abranches, P.; Rioux, J.-A.; Santos-Gomes, G.; Alves-Pires, C.; Cortes, S.; Ramada, J.; Cristovão, J.M.; Afonso, M.O.; et al. Leishmaniasis in Portugal: Enzymatic Polymorphism of Leishmania infantum based on Identification of 213 Strains. Trop. Med. Int. Health 2006, 11, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Control of the Leishmaniases: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010; WHO Technical Report Series 949; World Health Organization: Geneva, Switzerland, 2010; p. 186.
- Garrote, J.I.; Gutierrez, M.P.; Lopez Izquierdo, R.; Duenas, M.A.I.; Zarzosa, P.; Canavete, C.; El Bali, M.; Almaraz, A.; Miguel, A.; Bratos, A.; et al. Seroepidemiologic study of Leishmania infantum infection in Castilla-Leon, Spain. Am. J. Trop. Med. Hyg. 2004, 71, 403–406. [Google Scholar] [PubMed]
- Campino, L.; Maia, C. Epidemiologia das leishmanioses em Portugal. Acta Med. Port. 2010, 23, 859–864. [Google Scholar] [PubMed]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Tempone, A.G.; Martins de Oliveira, C.; Berlinck, R.G. Current approaches to discover marine antileishmanial natural products. Planta Med. 2011, 77, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Pecoul, B. “Manifesto” for advancing the control and elimination of neglected tropical diseases. PLoS Negl. Trop. Dis. 2010, 4, e718. [Google Scholar] [CrossRef]
- Maia, C.; Nunes, M.; Cristóvão, J.; Campino, L. Experimental canine leishmaniasis: Clinical, parasitological and serological follow-up. Acta Trop. 2010, 116, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Watts, K.R.; Tenney, K.; Crews, P. The structural diversity and promise of antiparasitic marine invertebrate-derived small molecules. Curr. Opin. Biotechnol. 2010, 21, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Sineiro, J.; Rubilar, M.; Sánchez, M.; Jerez, M.; Pinelo, M.; Costoya, N.; Núñez, M.J. Polyphenols from plant materials: Extraction and antioxidant power. Electron J. Environ. Agric. Food Chem. 2008, 7, 3210–3216. [Google Scholar]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Duval, B.; Shetty, K.; Thomas, W.H. Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. J. Appl. Phycol. 2000, 11, 559–566. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; De Paepe, D.; Baart, G.J.E.; de Cooman, L. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; de Brabanter, J.; de Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- De la Vega, M.; Díaz, E.; Vila, M.; León, R. Isolation of a new strain of Picochlorum sp. and characterization of its potential biotechnological applications. Biotechnol. Prog. 2011, 27, 1535–1543. [Google Scholar]
- Jeffrey, S.W. Paper-chromatographic separation of chlorophylls and carotenoids from marine algae. Biochem. J. 1961, 80, 336–342. [Google Scholar] [PubMed]
- Liu, D.; Shi, J.; Ibarra, A.C.; Kakuda, Y.; Xue, S.J. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. Lebensm. Wiss. Technol. 2006, 41, 1344–1349. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Ohsawa, I.; Konishi, F.; Hasegawa, T.; Kumamoto, S.; Suzuki, Y.; Ohta, S. Preventive effects of Chlorella on cognitive decline in age-dependent dementia model mice. Neur. Lett. 2009, 464, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L. Biorefinery as a promising approach to promote microalgae industry: An innovative framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Bioref. 2010, 4, 287–295. [Google Scholar] [CrossRef]
- Subhadra, B.G.; Edwards, M. Coproduct market analysis and water footprint of simulated commercial algal biorefineries. Appl. Energy 2011, 88, 3515–3523. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control 2010, 21, 1425–1435. [Google Scholar] [CrossRef]
- Mente, E.; Karalazos, V.; Karapanagiotidis, I.T.; Pita, C. Nutrition in organic aquaculture: An inquiry and a discourse. Aquac. Nutr. 2011, 17, 798–817. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Mozes, A.; Florindo, C.; Polo, C.; Duarte, C.V.; Custódio, L.; Varela, J. Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnol. Biofuels 2011, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Scheyer, T.; Romano, C.; Vojnov, A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Megías, C.; Pastor-Cavada, E.; Torres-Fuentes, C.; Girón-Calle, J.; Alaiz, M.; Jua, R.; Julio, P.; Javier, V. Chelating, antioxidant and antiproliferative activity of Vicia sativa polyphenol extracts. Eur. Food Res. Technol. 2009, 230, 353–359. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Naz, Q.; Ejaz, A.; Yilmaz, G.; Kan, Y.K.B.; Sener, B.; Choudhary, M.I. Antioxidant and anticholinesterase evaluation of selected Turkish Salvia species. Food Chem. 2007, 103, 1247–1254. [Google Scholar] [CrossRef]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, H.; Custódio, L.; Rodrigues, M.J.; De Sousa, C.B.; Oliveira, M.; Barreira, L.; Neng, N.D.R.; Nogueira, J.M.F.; Alrokayan, S.A.; Mouffouk, F.; et al. Biological Activities and Chemical Composition of Methanolic Extracts of Selected Autochthonous Microalgae Strains from the Red Sea. Mar. Drugs 2015, 13, 3531-3549. https://doi.org/10.3390/md13063531
Pereira H, Custódio L, Rodrigues MJ, De Sousa CB, Oliveira M, Barreira L, Neng NDR, Nogueira JMF, Alrokayan SA, Mouffouk F, et al. Biological Activities and Chemical Composition of Methanolic Extracts of Selected Autochthonous Microalgae Strains from the Red Sea. Marine Drugs. 2015; 13(6):3531-3549. https://doi.org/10.3390/md13063531
Chicago/Turabian StylePereira, Hugo, Luísa Custódio, Maria João Rodrigues, Carolina Bruno De Sousa, Marta Oliveira, Luísa Barreira, Nuno Da Rosa Neng, José Manuel Florêncio Nogueira, Salman A. Alrokayan, Fouzi Mouffouk, and et al. 2015. "Biological Activities and Chemical Composition of Methanolic Extracts of Selected Autochthonous Microalgae Strains from the Red Sea" Marine Drugs 13, no. 6: 3531-3549. https://doi.org/10.3390/md13063531







