Activation of p53 with Ilimaquinone and Ethylsmenoquinone, Marine Sponge Metabolites, Induces Apoptosis and Autophagy in Colon Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Ilimaquinone and Ethylsmenoquinone as Activators of the p53 Pathway
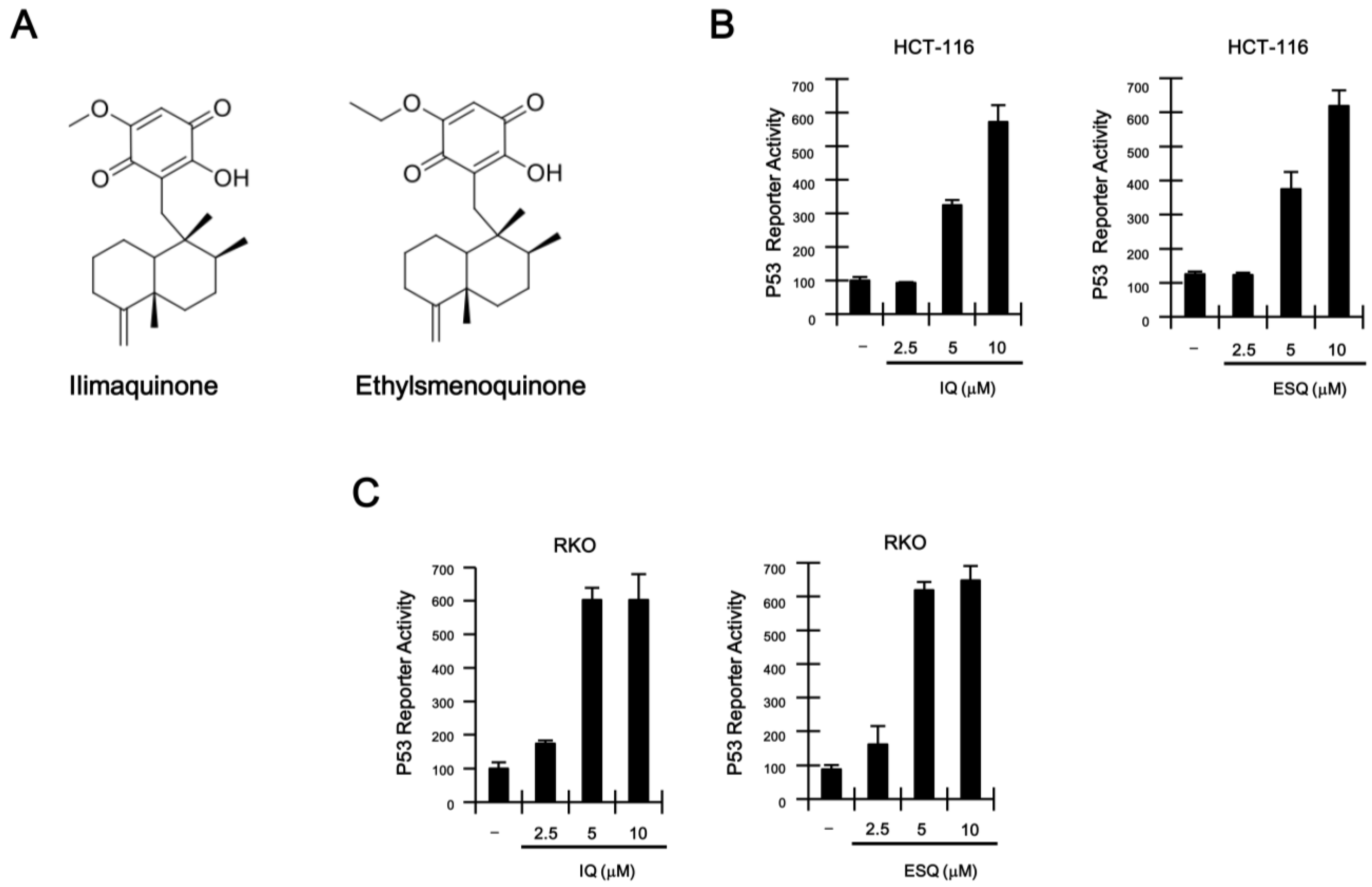


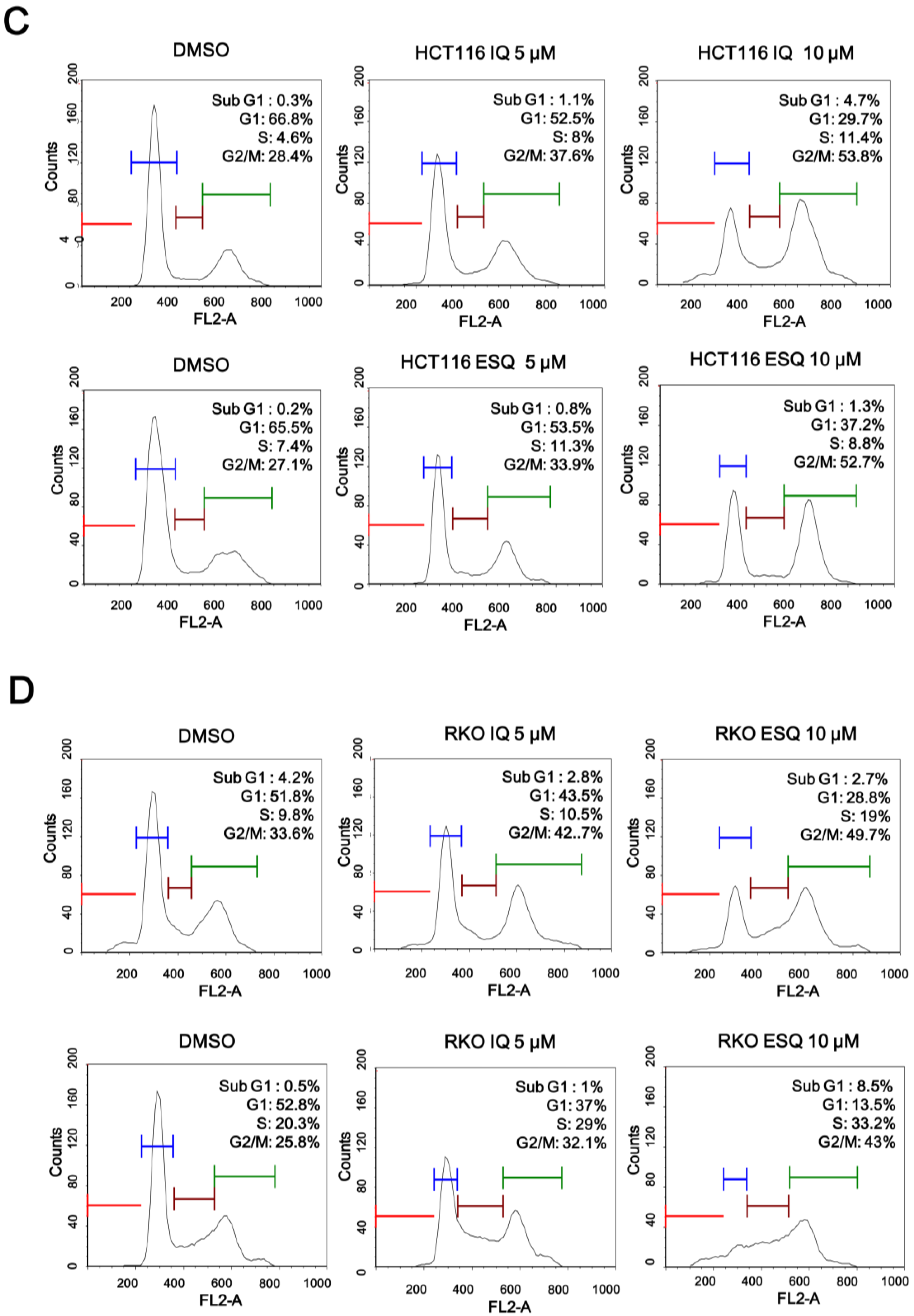
2.2. Ilimaquinone and Ethylsmenoquinone Induce G2/M Cell Cycle Arrest in Colon Cancer Cells
2.3. Ilimaquinone and Ethylsmenoquinone Induce Apoptosis in Colon Cancer Cells


2.4. Ilimaquinone and Ethylsmenoquinone Induce Autophagy in Colon Cancer Cells

3. Discussion
4. Experimental Section
4.1. Isolation of Ilimaquinone and Synthesis of Ethylsmenoquinone
4.2. Cell Culture, Transfection and Luciferase Assay
4.3. Screening for Small Molecule Activators of the p53 Pathway
4.4. Western Blot Analysis
4.5. RNA Extraction and Semi-Quantitative RT-PCR
4.6. Cell Viability Assay
4.7. Cell Cycle Analysis
4.8. Apoptosis Analysis
4.9. Confocal Microscopy of GFP-LC3 Fluorescence
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Levine, A.J.; Oren, M. The first 30 years of p53: Growing ever more complex. Nat. Rev. Cancer 2009, 9, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Rathmell, J.C. Rathmell. Metabolic Stress in Autophagy and Cell Death Pathways. Cold Spring Harb. Perspect. Biol 2012, 4, a008763. [Google Scholar]
- Sun, X.X.; Dai, M.S. Deubiquitinating enzyme regulation of the p53 pathway: A lesson from Otub1. World J. Biol. Chem. 2014, 5, 75–84. [Google Scholar] [PubMed]
- Oren, M.; Rotter, V. Mutant p53 Gain-of-Function in Cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a001107. [Google Scholar] [CrossRef] [PubMed]
- Hundley, J.E.; Koester, S.K.; Troyer, D.A.; Hilsenbeck, S.G.; Subler, M.A.; Windle, J.J. Increased tumor proliferation and genomic instability without decreased apoptosis in MMTV-rars mice deficient in p53. Mol. Cell. Biol. 1997, 17, 723–731. [Google Scholar] [PubMed]
- Michael, D.; Oren, M. The p53-Mdm2 module and the ubiquitin system. Semin. Cancer Biol. 2003, 13, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.L.; Gu, W. Ubiquitination, phosphorylation and acetylation: The molecular basis for p53 regulation. Curr. Opin. Cell Biol. 2003, 15, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Loughery, J.; Cox, M.; Smith, L.M.; Meek, D.W. Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic Acids Res. 2014, 42, 7666–7680. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Mak, T.W. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat. Rev. Cancer 2004, 4, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Speidel, D. Transcription-independent p53 apoptosis: An alternative route to death. Trends Cell Biol. 2010, 20, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Na, M.; Kim, H.G.; Khanal, T.; Choi, J.H.; Jin, S.W.; Oh, S.H.; Hwang, I.H.; Chung, Y.C.; Kim, H.S.; et al. Ilimaquinone induces death receptor expression and sensitizes human colon cancer cells to TRAIL-induced apoptosis through activation of ROS-ERK/p38 MAPK-CHOP signaling pathways. Food Chem. Toxicol. 2014, 71, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L. Mechanisms of transport through the Golgi complex. J. Cell Sci. 2009, 122, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhou, Y.D.; Nagle, D.G. Inducers of Hypoxic Response: Marine Sesquiterpene Quinones Activate HIF-1. J. Nat. Prod. 2013, 76, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.C.; Huang, S.Y.; Chen, S.P.; Su, C.C.; Chiu, T.L.; Pang, C.Y. Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis. 2013, 16, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yun, E.; Hwang, I.H.; Yoon, S.; Kim, D.E.; Kim, J.S.; Na, M.; Song, G.Y.; Oh, S. Ilimaquinone and Ethylsmenoquinone, Marine Sponge Metabolites, Suppress the Proliferation of Multiple Myeloma Cells by Down-Regulating the Level of β-Catenin. Mar. Drugs 2014, 12, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Oh, S.; Shin, S.; Janknecht, R. Regulation of Tumor Suppressor p53 and HCT116 Cell Physiology by Histone Demethylase JMJD2D/KDM4D. PLoS One 2012, 7, e34618. [Google Scholar] [CrossRef] [PubMed]
- Moos, P.J.; Edes, K.; Fitzpatrick, F.A. Inactivation of wild-type p53 tumor suppressor by electrophilic prostaglandins. Proc. Natl. Acad. Sci. USA 2000, 97, 9215–9220. [Google Scholar] [CrossRef] [PubMed]
- Jäämaa, S.; Af Hällström, T.M.; Sankila, A.; Rantanen, V.; Koistinen, H.; Stenman, U.H.; Zhang, Z.; Yang, Z.; de Marzo, A.M.; Taari, K.; et al. DNA Damage Recognition via Activated ATM and p53 Pathway in Nonproliferating Human Prostate Tissue. Cancer Res. 2010, 70, 8630–8641. [Google Scholar] [CrossRef] [PubMed]
- McLeland, C.B.; Rodriguez, J.; Stern, S.T. Autophagy monitoring assay: Qualitative analysis of MAP LC3-I to II conversion by immunoblot. Methods Mol. Biol. 2011, 697, 199–206. [Google Scholar] [PubMed]
- Haggar, F.A.; Boushey, R.P. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, Y.; Hamrick, H.E.; Duerksen-Hughes, P.J. ATM, ATR and DNA-PK: Initiators of the cellular genotoxic stress responses. Carcinogenesis 2003, 24, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, R.; Suico, M.A.; Koyama, K.; Omachi, K.; Kai, Y.; Matsuyama, S.; Mitsutake, K.; Taura, M.; Morino-Koga, S.; Shuto, T.; et al. Mild Electrical Stimulation at 0.1-ms Pulse Width Induces p53 Protein Phosphorylation and G2 Arrest in Human Epithelial Cells. J. Biol. Chem. 2013, 288, 16117–16126. [Google Scholar] [CrossRef] [PubMed]
- Brancho, D.; Tanaka, N.; Jaeschke, A.; Ventura, J.J.; Kelkar, N.; Tanaka, Y.; Kyuuma, M.; Takeshita, T.; Flavell, R.A.; Davis, R.J. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003, 17, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.N.; Tolkovsky, A.M. A Role for MAPK/ERK in Sympathetic Neuron Survival: Protection against a p53-Dependent, JNK-Independent Induction of Apoptosis by Cytosine Arabinoside. J. Neurosci. 1999, 19, 664–673. [Google Scholar] [PubMed]
- Nieminen, A.I.; Eskelinen, V.M.; Haikala, H.M.; Tervonen, T.A.; Yan, Y.; Partanen, J.I.; Klefström, J. Myc-induced AMPK-phospho p53 pathway activates Bak to sensitize mitochondrial apoptosis. Proc. Natl. Acad. Sci. USA 2013, 110, E1839–E1848. [Google Scholar] [CrossRef] [PubMed]
- Amado, N.G.; Predes, D.; Fonseca, B.F.; Cerqueira, D.M.; Reis, A.H.; Dudenhoeffer, A.C.; Borges, H.L.; Mendes, F.A.; Abreu, J.G. Isoquercitrin Suppresses Colon Cancer Cell Growth in Vitro by Targeting the Wnt/β-Catenin Signaling Pathway. J. Biol. Chem. 2014, 289, 35456–35467. [Google Scholar] [CrossRef] [PubMed]
- Roura, S.; Martínez, D.; Piedra, J.; Miravet, S.; García de Herreros, A.; Duñach, M. APC 3 × 15 beta-catenin-binding domain potentiates beta-catenin association to TBP and upregulates TCF-4 transcriptional activity. Biochem. Biophys. Res. Commun. 2003, 309, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Korinek, V.; Barker, N.; Morin, P.J.; van Wichen, D.; de Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/-colon carcinoma. Science 1997, 275, 1784–1787. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.H.; Song, G.Y.; Kim, D.E.; Jeong, Y.J.; Liu, K.H.; Chung, Y.H.; Oh, S. Ursolic acid and its natural derivative corosolic acid suppress the proliferation of APC-mutated colon cancer cells through promotion of β-catenin degradation. Food Chem. Toxico. 2014, 67, 87–95. [Google Scholar] [CrossRef]
- Thanendrarajan, S.; Kim, Y.; Schmidt-Wolf, I.G. Understanding and Targeting the Wnt/β-Catenin Signaling Pathway in Chronic Leukemia. Leuk. Res. Treat. 2011, 2011. [Google Scholar] [CrossRef]
- Harris, S.L.; Levine, A.J. The p53 pathway: Positive and negative feedback loops. Oncogene 2005, 24, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Knibiehler, M.; Ducommun, B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene 1998, 16, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.J.; Zhivotovsky, B. DNA damage-induced apoptosis. Oncogene 2004, 23, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Tzifi, F.; Economopoulou, C.; Gourgiotis, D.; Ardavanis, A.; Papageorgiou, S.; Scorilas, A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv. Hematol. 2012, 2012. [Google Scholar] [CrossRef]
- Gump, J.M.; Thorburn, A. Autophagy and apoptosis: What is the connection? Trends Cell Biol. 2011, 21, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Livesey, K.M.; Kang, R.; Vernon, P.; Buchser, W.; Loughran, P.; Watkins, S.C.; Zhang, L.; Manfredi, J.J.; Zeh, H.J., 3rd; Li, L.; et al. p53/HMGB1 Complexes Regulate Autophagy and Apoptosis. Cancer Res. 2012, 72, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska, A.J.; Rao, K.V.; Childress, S.; El-Alfy, A.; Matsumoto, R.R.; Kelly, M.; Stewart, G.S.; Sufka, K.J.; Hamann, M.T. Secondary Metabolites from Three Florida Sponges with Antidepressant Activity. J. Nat. Prod. 2008, 71, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.K.; Gwak, J.; Song, I.S.; Park, H.S.; Oh, S. Induction of apoptosis in colon cancer cells by a novel topoisomerase I inhibitor TopIn. Biochem. Biophys. Res. Commun. 2011, 409, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Gwak, J.; Cho, M.; Song, T.; Won, J.; Kim, D.E.; Shin, J.G.; Oh, S. Hexachlorophene inhibits Wnt/β-catenin pathway by promoting Siah-mediated β-catenin degradation. Mol. Pharmacol. 2006, 70, 960–966. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Chung, K.J.; Hwang, I.H.; Gwak, J.; Park, S.; Ju, B.G.; Yun, E.; Kim, D.-E.; Chung, Y.-H.; Na, M.; et al. Activation of p53 with Ilimaquinone and Ethylsmenoquinone, Marine Sponge Metabolites, Induces Apoptosis and Autophagy in Colon Cancer Cells. Mar. Drugs 2015, 13, 543-557. https://doi.org/10.3390/md13010543
Lee H-Y, Chung KJ, Hwang IH, Gwak J, Park S, Ju BG, Yun E, Kim D-E, Chung Y-H, Na M, et al. Activation of p53 with Ilimaquinone and Ethylsmenoquinone, Marine Sponge Metabolites, Induces Apoptosis and Autophagy in Colon Cancer Cells. Marine Drugs. 2015; 13(1):543-557. https://doi.org/10.3390/md13010543
Chicago/Turabian StyleLee, Hyun-Young, Kyu Jin Chung, In Hyun Hwang, Jungsuk Gwak, Seoyoung Park, Bong Gun Ju, Eunju Yun, Dong-Eun Kim, Young-Hwa Chung, MinKyun Na, and et al. 2015. "Activation of p53 with Ilimaquinone and Ethylsmenoquinone, Marine Sponge Metabolites, Induces Apoptosis and Autophagy in Colon Cancer Cells" Marine Drugs 13, no. 1: 543-557. https://doi.org/10.3390/md13010543




