Porphyra-334 Isolated from the Marine Algae Bangia atropurpurea: Conformational Performance for Energy Conversion
Abstract
:1. Introduction
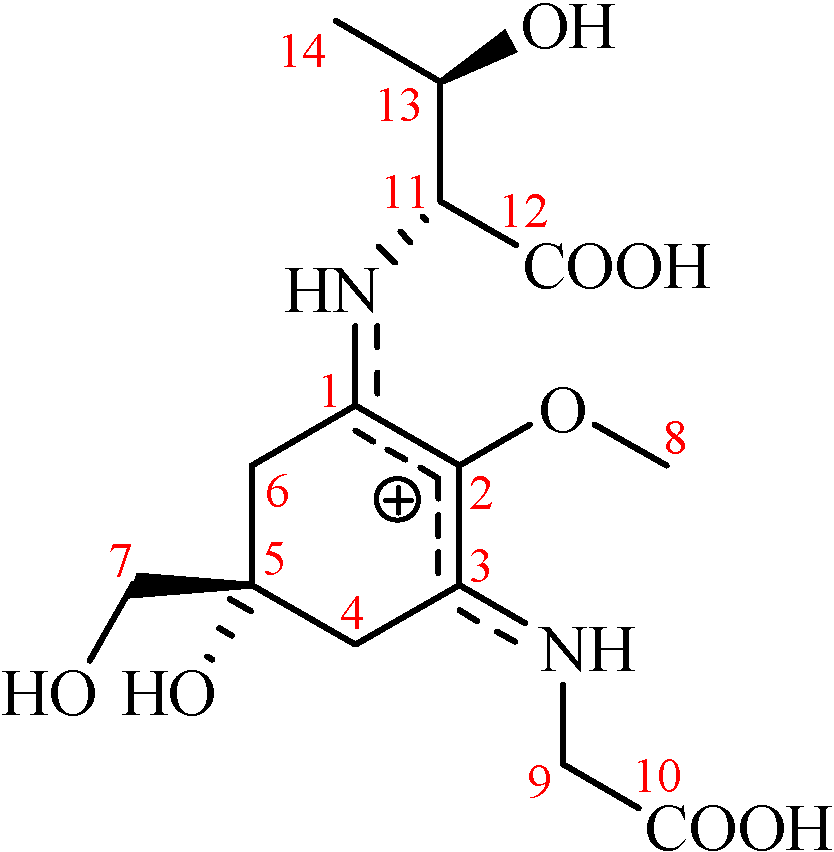
2. Results and Discussion
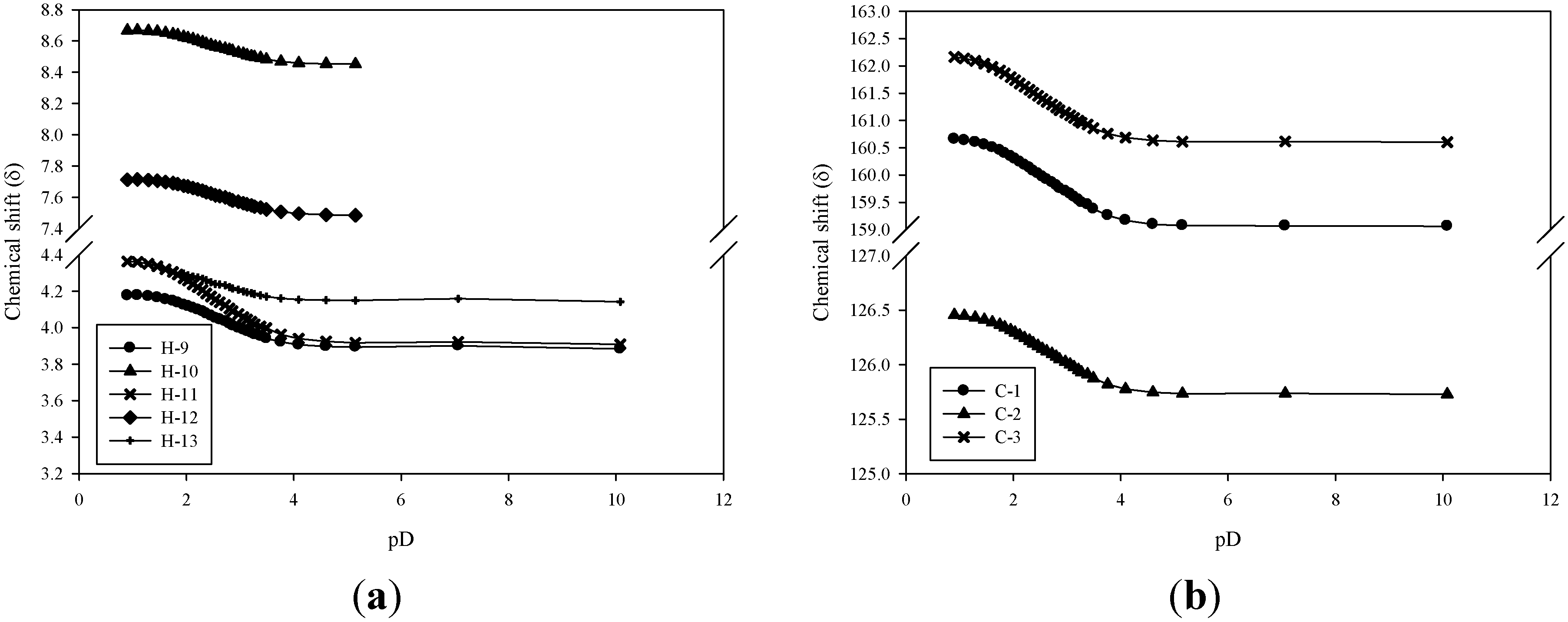

| pD | 1.0 | 2.0 | 3.5 | |||
|---|---|---|---|---|---|---|
| Proton | Oxygen | (Å) | Oxygen | (Å) | Oxygen | (Å) |
| C(1)N-H a | C(2)O | 2.21 | C(2)O | 2.20 | C(12)O | 1.64 |
| C(13)O | 1.98 | C(13)O | 2.44 | - | - | |
| C(3)N-H b | C(2)O | 2.14 | C(2)O | 2.30 | - | - |
| C(10)O | 2.09 | C(10)O | 2.58 | - | - | |
| C(5)O-H | C(7)O | 2.15 | C(7)O | 2.11 | - | - |
| H2-9 | C(10)O | 2.61 | C(10)O | 2.58 | C(10)O | 2.60 |
| C(10)O-H | - | - | - | - | C(2)O | 2.09 |
| H-11 | C(5)O | 2.50 | C(5)O | 2.45 | C(12)O | 2.08 |
| C(12)O | 2.70 | C(13)O | 2.55 | C(13)O | 2.61 | |
| H-13 | C(2)O | 2.64 | C(12)O | 2.77 | C(12)O | 2.44 |
| C(13)O | 2.01 | C(13)O | 2.04 | C(13)O | 2.03 | |
| C(13)O-H | - | - | - | - | C(12)O | 1.85 |
| H3-14 | C(12)O | 2.57 | C(13)O | 2.64 | C(2)O | 2.61 |
| C(13)O | 2.63 | - | - | C(13)O | 2.59 | |
3. Experimental Section
3.1. Procedure for Purifying P-334
3.2. Properties of the P-334 Experiment
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sinha, R.P.; Singh, S.P.; Häder, D.P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Sawall, T.; Wiencke, C. Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar. Ecol. Prog. Ser. 2001, 211, 117–129. [Google Scholar] [CrossRef]
- Huovinen, P.; Gómez, I.; Figueroa, F.L.; Ulloa, N.; Morales, V.; Lovengreen, C. Ultraviolet-absorbing mycosporine-like amino acids in red macroalgae from Chile. Bot. Mar. 2004, 47, 21–29. [Google Scholar]
- Oyamada, C.; Kaneniwa, M.; Ebitani, K.; Murata, M.; Ishihara, K. Mycosporine-like amino acids extracted from scallop (Patinopecten yessoensis) ovaries: UV protection and growth stimulation activities on human cells. Mar. Biotechnol. 2008, 10, 141–150. [Google Scholar] [CrossRef]
- Tao, C.; Sugawara, T.; Maeda, S.; Wang, X.; Hirata, T. Antioxidative activities of a mycosporine-like amino acid, porphyra-334. Fish. Sci. 2008, 74, 1161–1172. [Google Scholar]
- Torres, A.; Enk, C.D.; Hochberg, M.; Srebnik, M. Porphyra-334, a potential natural source for UVA protective sunscreens. Photochem. Photobiol. Sci. 2006, 5, 432–435. [Google Scholar]
- Cardozo, K.H.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B 2000, 56, 139–144. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar] [CrossRef]
- Zhang, L.W.; Tashiro, U.; Matsukawa, S.; Ogawa, H. Influence of pH and temperature on the ultraviolet-absorbing properties of porphyra-334. Fish. Sci. 2005, 71, 1382–1384. [Google Scholar] [CrossRef]
- Klisch, M.; Richter, P.; Puchta, R.; Hader, D.P.; Bauer, W. The stereostructure of porphyra-334: An experimental and calculational NMR investigation. Evidence for an efficient “proton sponge”. Helv. Chim. Acta 2007, 90, 488–511. [Google Scholar] [CrossRef]
- Kakehashi, R.; Shizuma, M.; Yamamura, S.; Maeda, H. Hydrogen ion titration of alkyldimethylamine oxides by 13C and 1H NMR and conventional methods. J. Colloid Interface Sci. 2005, 289, 498–503. [Google Scholar] [CrossRef]
- Krężel, A.; Bal, W. A formula for correlating pKa values determined in D2O and H2O. J. Inorg. Biochem. 2004, 98, 161–166. [Google Scholar] [CrossRef]
- Weinberg, D.R.; Gagliardi, C.J.; Hull, J.F.; Murphy, C.F.; Kent, C.A.; Westlake, B.C.; Paul, A.; Ess, D.H.; McCafferty, D.G.; Meyer, T.J. Proton-coupled electron transfer. Chem. Rev. 2012, 112, 4016–4093. [Google Scholar] [CrossRef]
- Cunningham, M.L.; Johnson, J.S.; Giovanazzi, S.M.; Peak, M.J. Photosensitized production of superoxide anion by monochromatic (290–405 nm) ultraviolet irradiation of NADH and NADPH coenzymes. Photochem. Photobiol. 1985, 42, 125–128. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef]
- Wenthold, P.G.; Squires, R.R. Biradical thermochemistry from collision-induced dissociation threshold energy measurements-Absolute heats of formation of ortho-benzyne, meta-benzyne, and para-benzyne. J. Am. Chem. Soc. 1994, 116, 6401–6412. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Ellison, G.B. Bond dissociation energies of organic molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef]
- Wenthold, P.G.; Squires, R.R. Gas-phase properties and reactivity of the acetate radical-anion-determination of the C–H bond strengths in acetic-acid and acetate ion. J. Am. Chem. Soc. 1994, 116, 11890–11897. [Google Scholar] [CrossRef]
- Schulten, K.; Tavan, P. A mechanism for the light-driven proton pump of Halobacterium halobium. Nature 1978, 272, 85–86. [Google Scholar]
- Kouyama, T.; Kouyama, A.N.; Ikegami, A. Bacteriorhodopsin is a powerful light-driven proton pump. Biophys. J. 1987, 51, 839–841. [Google Scholar] [CrossRef]
- Tributsch, H. Light driven proton pumps. Ionics 2000, 6, 161–171. [Google Scholar] [CrossRef]
- Oren, A. Mycosporine-like amino acids as osmotic solutes in a community of halophilic cyanobacteria. Geomicrobiol. J. 1997, 14, 231–240. [Google Scholar] [CrossRef]
- Portwich, A.; Garcia-Pichel, F. Ultraviolet and osmotic stresses induce and regulate the synthesis of mycosporines in the cyanobacterium Chlorogloeopsis PCC 6912. Arch. Microbiol. 1999, 172, 187–192. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Williams, D.McB.; Chalker, B.E.; Banaszak, A.T. Biochemical photoadaptations in vision: UV-absorbing pigments in fish eye tissues. Comp. Biochem. Physiol. B 1989, 93, 601–607. [Google Scholar] [CrossRef]
- Mason, D.S.; Schafer, F.; Shick, J.M.; Dunlap, W.C. Ultraviolet radiation-absorbing mycosporine-like amino acids (MAAs) are acquired from their diet by medaka fish (Oryzias latipes) but not by SKH-1 hairless mice. Comp. Biochem. Physiol. A 1998, 120, 587–598. [Google Scholar]
- Adams, N.L.; Shick, J.M. Mycosporine-like amino acids prevent UVB-induced abnormalities during early development of the green sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 2001, 138, 267–280. [Google Scholar] [CrossRef]
- Adams, N.L.; Shick, J.M.; Dunlap, W.C. Selective accumulation of mycosporine-like amino acids in ovaries of the green sea urchin Strongylocentrotus droebachiensis is not affected by ultraviolet radiation. Mar. Biol. 2001, 138, 281–294. [Google Scholar] [CrossRef]
- Karentz, D.; Dunlap, W.C.; Bosch, I. Temporal and spatial occurrence of UV-absorbing mycosporine-like amino acids in tissues of the antarctic sea urchin Sterechinus neumayeri during springtime ozone-depletion. Mar. Biol. 1997, 129, 343–353. [Google Scholar]
- Grant, P.T.; Plack, P.A. Gadusol, a metabolite from fish eggs. Tetrahedron Lett. 1980, 21, 4043–4044. [Google Scholar] [CrossRef]
- Plack, P.A.; Fraser, N.W.; Grant, P.T.; Middleton, C.; Mitchell, A.I.; Thomson, R.H. Gadusol, an enolic derivative of cyclohexane-1,3-dione present in the roes of cod and other marine fish. Isolation, properties and occurrence compared with ascorbic acid. Biochem. J. 1981, 199, 741–747. [Google Scholar]
- Volkmann, M.; Gorbushina, A.A. A broadly applicable method for extraction and characterization of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiol. Lett. 2006, 255, 286–295. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chuang, L.-F.; Chou, H.-N.; Sung, P.-J. Porphyra-334 Isolated from the Marine Algae Bangia atropurpurea: Conformational Performance for Energy Conversion. Mar. Drugs 2014, 12, 4732-4740. https://doi.org/10.3390/md12094732
Chuang L-F, Chou H-N, Sung P-J. Porphyra-334 Isolated from the Marine Algae Bangia atropurpurea: Conformational Performance for Energy Conversion. Marine Drugs. 2014; 12(9):4732-4740. https://doi.org/10.3390/md12094732
Chicago/Turabian StyleChuang, Li-Fan, Hong-Nong Chou, and Ping-Jyun Sung. 2014. "Porphyra-334 Isolated from the Marine Algae Bangia atropurpurea: Conformational Performance for Energy Conversion" Marine Drugs 12, no. 9: 4732-4740. https://doi.org/10.3390/md12094732





