Secondary Metabolites from the Soft Coral Sinularia arborea
Abstract
:1. Introduction

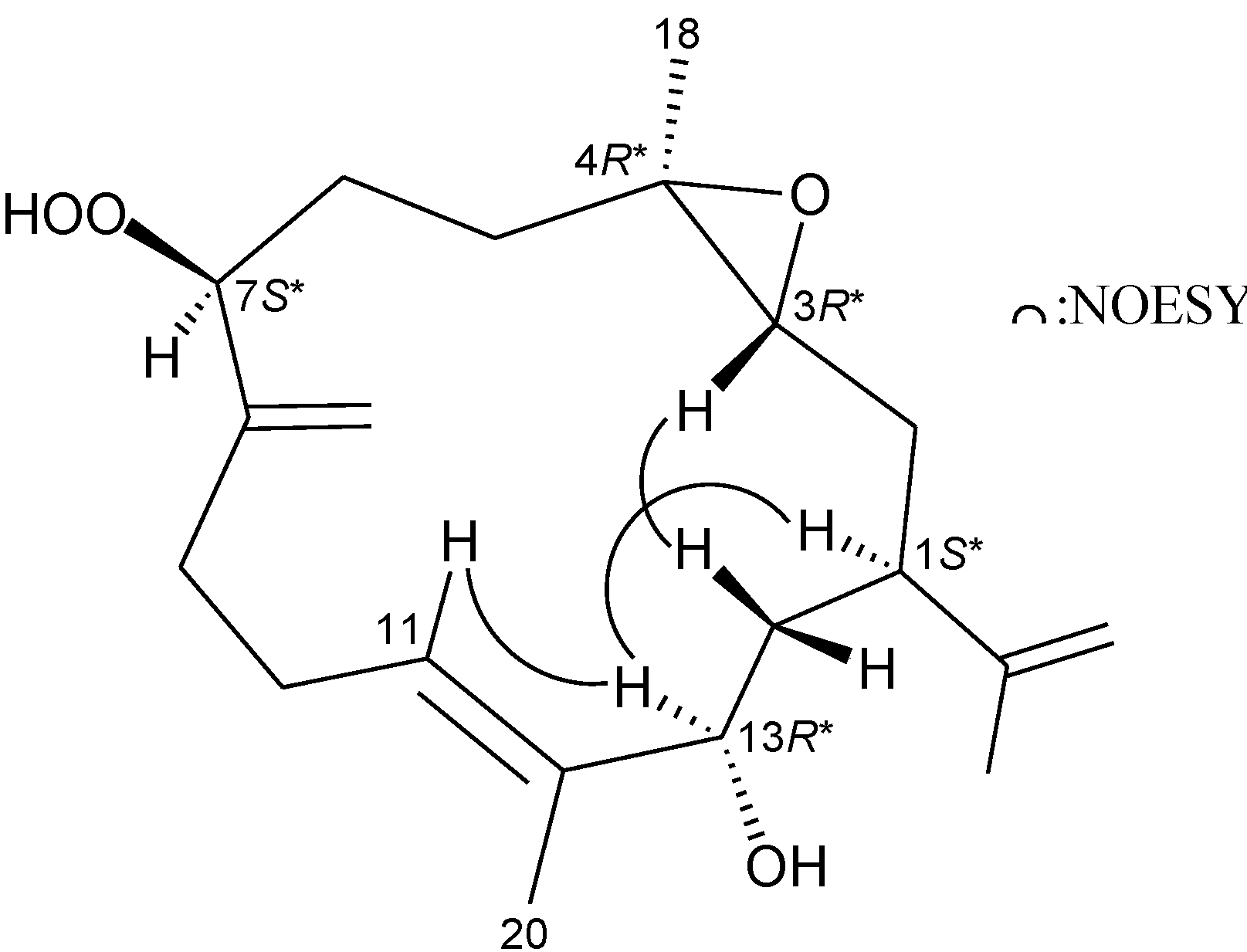
2. Results and Discussion
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.02 m | 43.1, CH | H2-2, H2-14 | C-2, -3, -13, -15, -17 |
| 2 | 1.89 m; 1.23 m | 32.9, CH2 | H-1, H-3 | C-1, -3, -4, -14, -15 |
| 3 | 2.72 dd (6.0, 6.0) | 61.4, CH | H2-2 | C-2 |
| 4 | 61.1, C | |||
| 5 | 1.96 m; 1.30 m | 33.7, CH2 | H2-6 | C-3, -4, -6, -7, -18 |
| 6 | 1.66 m; 1.52 m | 28.6, CH2 | H2-5, H-7 | C-4, -5, -7, -8 |
| 7 | 4.38 dd (7.2, 4.8) | 88.4, CH | H2-6 | C-5, -6, -19 |
| 8 | 147.3, C | |||
| 9 | 2.24 m | 31.3, CH2 | H2-10, H2-19 | C-8, -10, -11 |
| 10 | 2.41 m; 2.27 m | 25.9, CH2 | H2-9, H-11 | C-9 |
| 11 | 5.48 br s | 125.3, CH | H2-10, H3-20 | n.o. a |
| 12 | 138.4, C | |||
| 13 | 3.92 dd (6.0, 6.0) | 76.4, CH | H2-14 | C-1, -12, -14, -20 |
| 14 | 1.80 dd (6.8, 6.0) | 38.2, CH2 | H-1, H-13 | C-1, -2, -12, -13, -15 |
| 15 | 148.1, C | |||
| 16 | 1.71 br s | 18.8, CH3 | H2-17 | C-1, -15, -17 |
| 17 | 4.75 br s | 111.2, CH2 | H3-16 | C-1, -15, -16 |
| 18 | 1.27 s | 17.0, CH3 | C-3, -4, -5 | |
| 19 | 5.19 s; 5.15 s | 113.2, CH2 | H2-9 | C-7, -8, -9 |
| 20 | 1.69 s | 13.6, CH3 | H-11 | C-11, -12, -13 |
| 7-OOH | 7.89 br s | n.o. |

| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
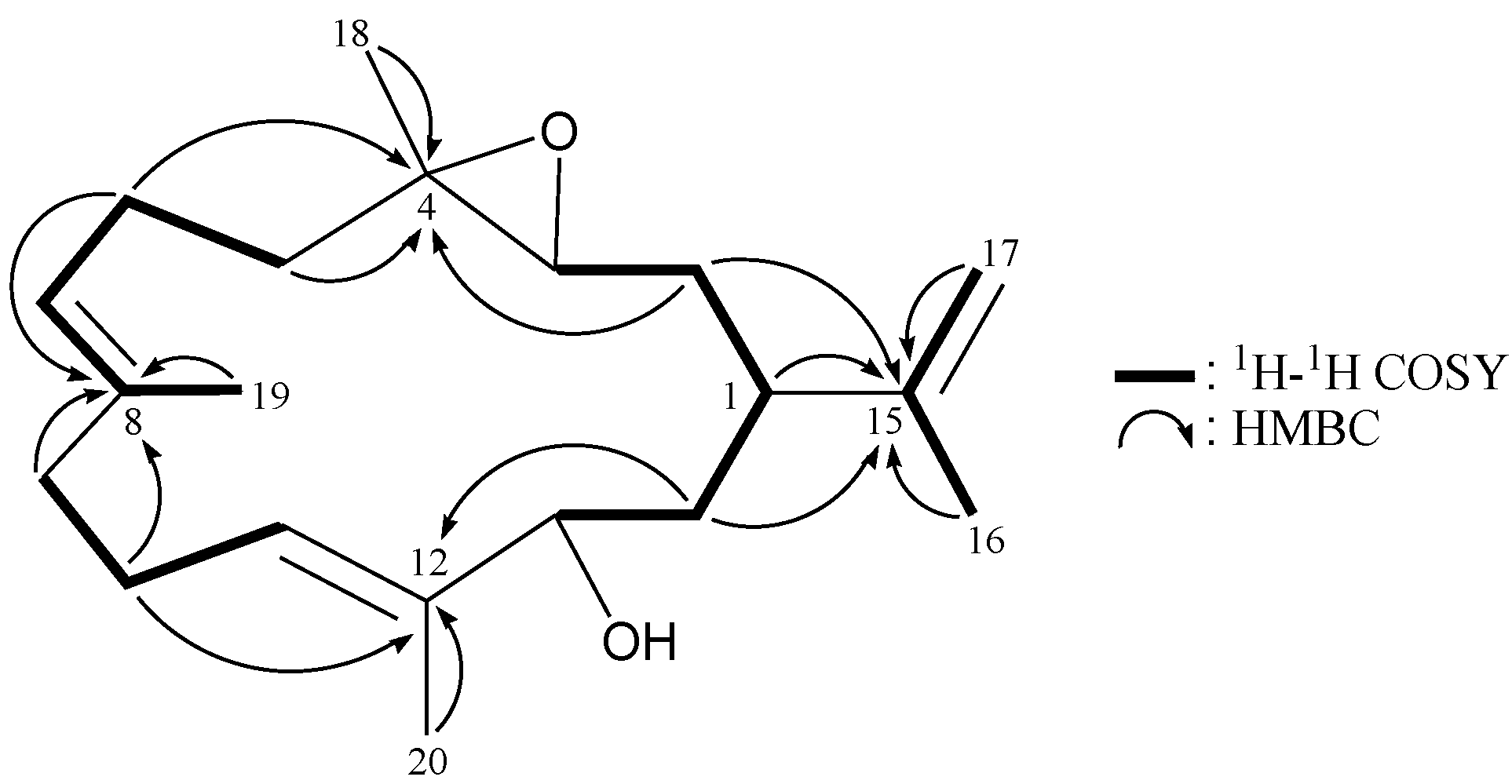
| 1 | 2.01 m | 40.7, CH | H2-2, H2-14 | C-3, -13, -15 |
| 2 | 1.77 m; 1.31 m | 33.5, CH2 | H-1, H-3 | C-1, -3, -4, -14, -15 |
| 3 | 2.74 dd (10.0, 2.8) | 63.3, CH | H2-2 | C-2 |
| 4 | 60.6, C | |||
| 5 | 2.06 m; 1.27 m | 38.1, CH2 | H2-6 | C-3, -4, -6, -7 |
| 6 | 2.29 m; 2.10 m | 24.0, CH2 | H2-5, H-7 | C-4, -5, -7, -8 |
| 7 | 5.05 ddq (6.4, 6.4, 1.2) | 123.2, CH | H2-6, H3-19 | C-6, -9, -19 |
| 8 | 135.1, C | |||
| 9 | 2.20 m; 2.04 m | 38.8, CH2 | H2-10 | C-7, -8, -10, -11, -19 |
| 10 | 2.16 m | 24.1, CH2 | H2-9, H-11 | C-8, -9, -11, -12 |
| 11 | 5.34 dd (6.4, 6.4) | 127.2, CH | H2-10 | C-9, -10, -13, -20 |
| 12 | 135.8, C | |||
| 13 | 3.87 br d (10.0) | 75.3, CH | H2-14 | C-11, -20 |
| 14 | 1.82 ddd (13.6, 10.8, 2.8) 1.73 ddd (13.6, 10.0, 3.6) | 37.3, CH2 | H-1, H-13 | C-1, -2, -12, -15 |
| 15 | 147.6, C | |||
| 16 | 1.67 br s | 18.5, CH3 | H2-17 | C-1, -15, -17 |
| 17 | 4.71 dd (2.0, 1.6); 4.64 dd (1.6, 0.8) | 111.0, CH2 | H3-16 | C-1, -15, -16 |
| 18 | 1.23 s | 16.7, CH3 | C-3, -4, -5 | |
| 19 | 1.63 br s | 16.2, CH3 | H-7 | C-7, -8, -9 |
| 20 | 1.67 s | 13.0, CH3 | C-11, -12, -13 |

| Cell lines IC50 (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| K562 | MOLT-4 | HTC-116 | DLD-1 | T-47D | MDA-MB-231 | MCF-7 | |
| 1 | NA | NA | NA | NA | NA | NA | NA |
| 2 | NA | 19.0 | NA | NA | NA | NA | NA |
| 3 | 2.5 | 0.7 | 19.0 | NA | NA | NA | NA |
| Doxorubicin a | 0.3 | 0.001 | 0.06 | 1.1 | 0.3 | 0.4 | 10.0 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
 +12 (c 0.15, CHCl3); IR (neat) νmax 3445 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1; ESIMS: m/z 359 [M + Na]+; HRESIMS: m/z 359.2195 (calcd for C20H32O4Na, 359.2198).
+12 (c 0.15, CHCl3); IR (neat) νmax 3445 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1; ESIMS: m/z 359 [M + Na]+; HRESIMS: m/z 359.2195 (calcd for C20H32O4Na, 359.2198). −3 (c 0.19, CHCl3); IR (neat) νmax 3419 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2; ESIMS: m/z 327 [M + Na]+; HRESIMS: m/z 327.2298 (calcd for C20H32O2Na, 327.2300).
−3 (c 0.19, CHCl3); IR (neat) νmax 3419 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2; ESIMS: m/z 327 [M + Na]+; HRESIMS: m/z 327.2298 (calcd for C20H32O2Na, 327.2300).3.4. Cytotoxicity Testing
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chen, W.-T.; Li, Y.; Guo, Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–237. [Google Scholar]
- Yang, B.; Zhou, X.-F.; Lin, X.-P.; Liu, J.; Peng, Y.; Yang, X.-W.; Liu, Y. Cembrane diterpenes chemistry and biological properties. Curr. Org. Chem. 2012, 16, 1512–1539. [Google Scholar] [CrossRef]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a source of new marine bioactive compounds—an overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef]
- Chao, C.-H.; Chou, K.-J.; Huang, C.-Y.; Wen, Z.-H.; Hsu, C.-H.; Wu, Y.-C.; Dai, C.-F.; Sheu, J.-H. Steroids from the soft coral Sinularia crassa. Mar. Drugs 2012, 10, 439–450. [Google Scholar] [CrossRef]
- Su, J.-H.; Ahmed, A.F.; Sung, P.-J.; Chao, C.-H.; Kuo, Y.-H.; Sheu, J.-H. Manaarenolides A–I, diterpenoids from the soft coral Sinularia manaarensis. J. Nat. Prod. 2006, 69, 1134–1139. [Google Scholar] [CrossRef]
- Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Irace, C.; Maffettone, C.; Bavestrello, G.; Cerrano, C. Oxygenated cembranoids of the decaryiol type from the Indonesian soft coral Lobophytum sp. Tetrahedron 2009, 65, 2898–2904. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Lu, Y.; Su, J.-H.; Wen, Z.-H.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Bioactive cembranoids from the Dongsha atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2011, 9, 994–1006. [Google Scholar] [CrossRef]
- Verseveldt, J. Octocorallia from North-Western Madagascar (Part II). Zool. Verh. 1971, 117, 1–73. [Google Scholar]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, K.-H.; Dai, C.-F.; Lu, M.-C.; Li, J.-J.; Chen, J.-J.; Chang, Y.-C.; Su, Y.-D.; Wang, W.-H.; Sung, P.-J. Secondary Metabolites from the Soft Coral Sinularia arborea. Mar. Drugs 2013, 11, 3372-3380. https://doi.org/10.3390/md11093372
Chen K-H, Dai C-F, Lu M-C, Li J-J, Chen J-J, Chang Y-C, Su Y-D, Wang W-H, Sung P-J. Secondary Metabolites from the Soft Coral Sinularia arborea. Marine Drugs. 2013; 11(9):3372-3380. https://doi.org/10.3390/md11093372
Chicago/Turabian StyleChen, Kuan-Hua, Chang-Feng Dai, Mei-Chin Lu, Jan-Jung Li, Jih-Jung Chen, Yu-Chia Chang, Yin-Di Su, Wei-Hsien Wang, and Ping-Jyun Sung. 2013. "Secondary Metabolites from the Soft Coral Sinularia arborea" Marine Drugs 11, no. 9: 3372-3380. https://doi.org/10.3390/md11093372






